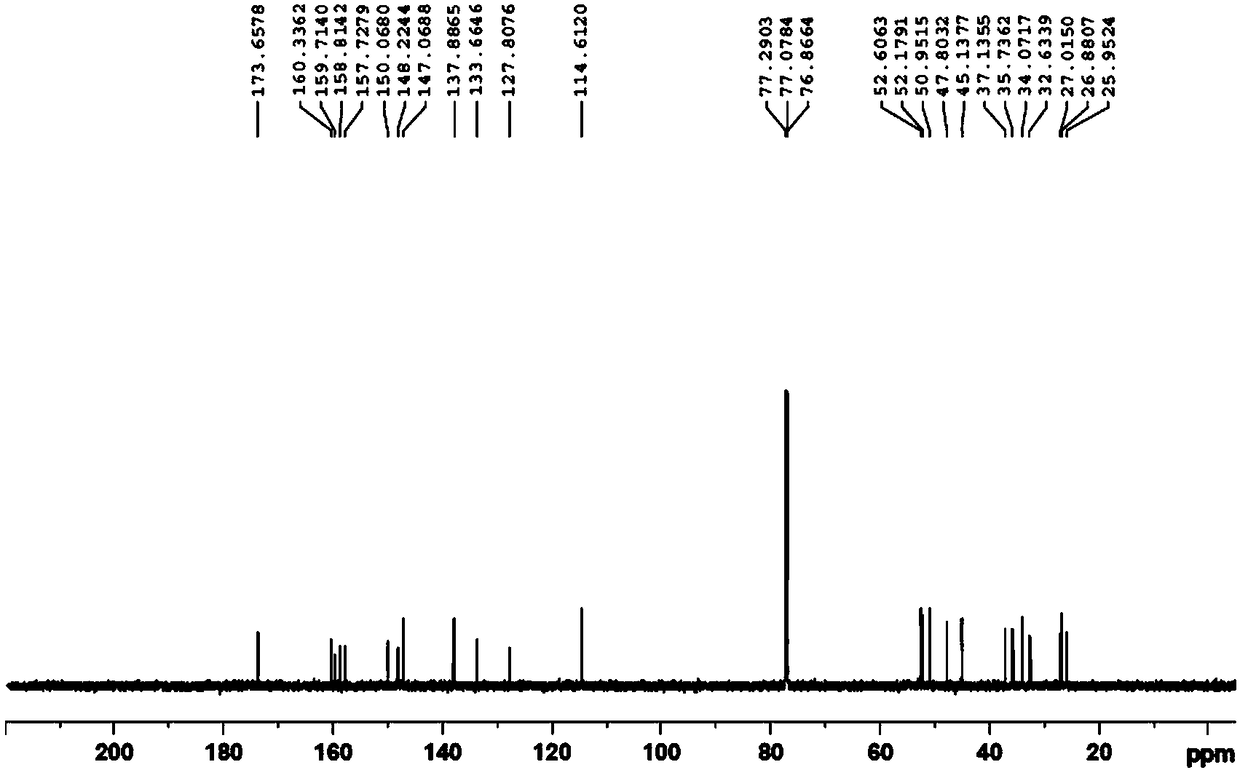
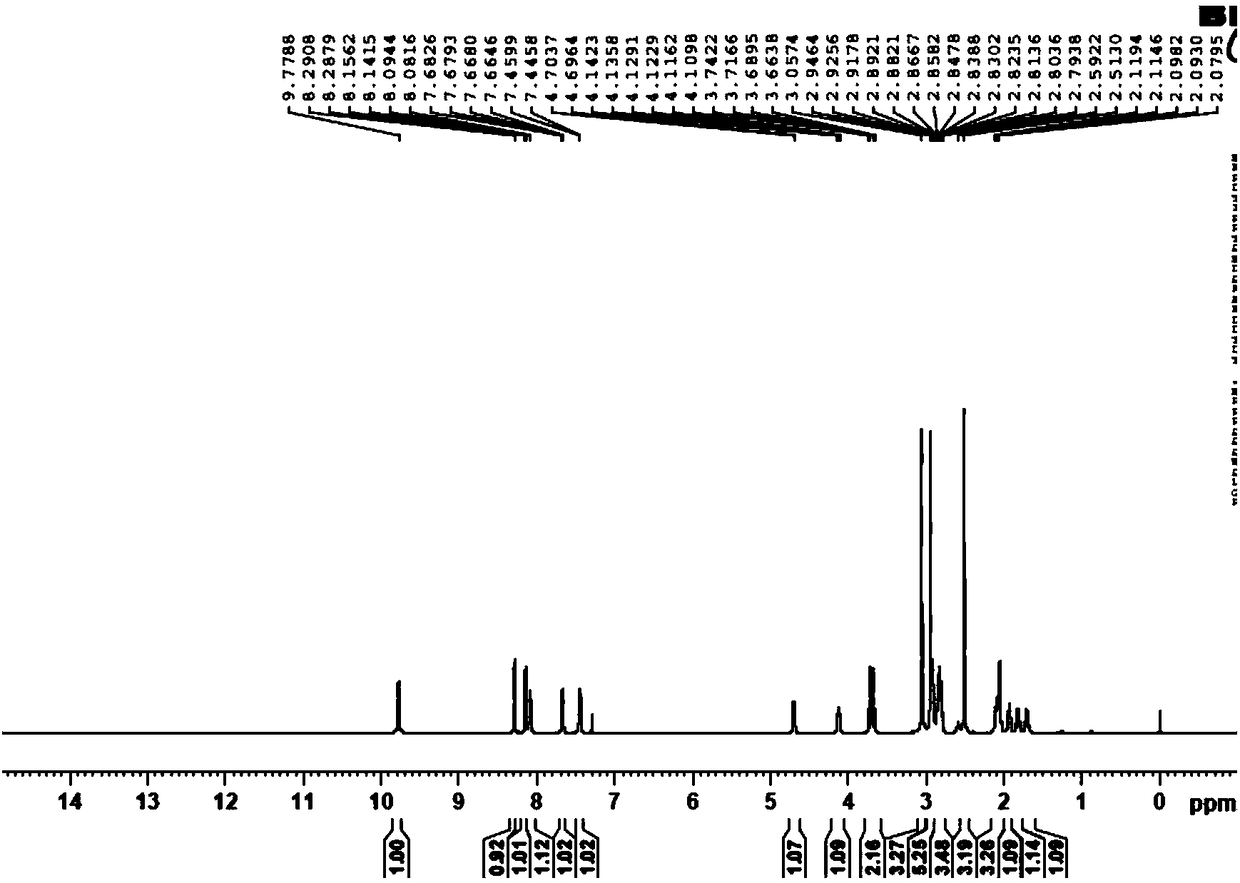
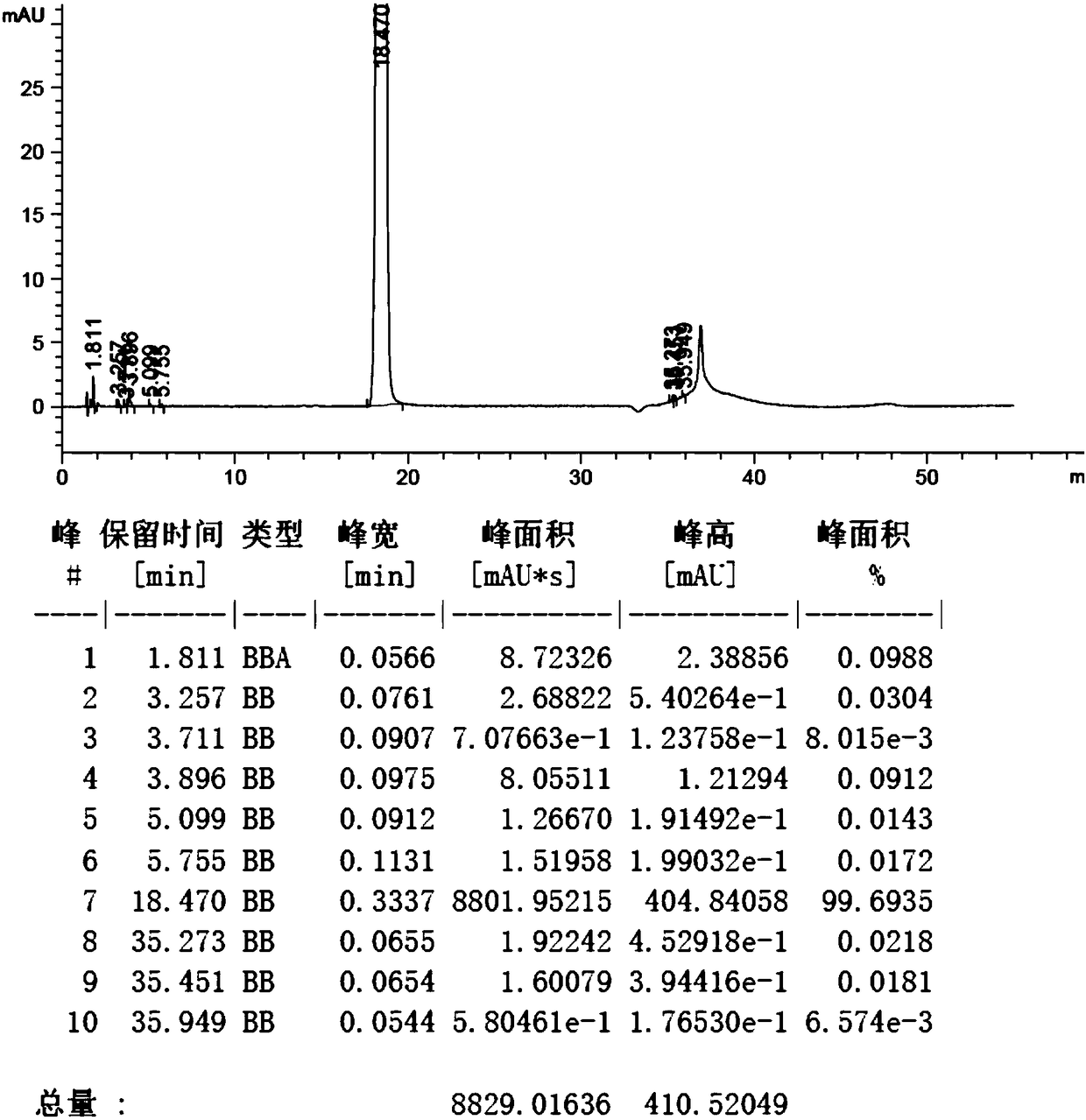
Preparation method of edoxaban p-toluenesulfonate monohydrate
A technology of p-toluenesulfonate and p-toluenesulfonic acid, which is applied in the field of preparation of edoxaban p-toluenesulfonate monohydrate, can solve the problems of low product purity, complicated process, and low total yield, and achieve The effect of promoting rapid reaction, increasing yield and product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0049] 1. Preparation of DXB-AC
[0050] Add 2.1g of 1-butyl-3-methylimidazole hydroxide, 100.0g of DXB-A, 85g of DXB-C and 600ml of acetonitrile into the three-necked flask, stir, add 113g of triethylamine and 21g of pyridine, stir the turbid solution, and start Raise the temperature and stir. When the temperature of the system rises to 45-60°C, the system becomes viscous and the stirring is difficult. Add 100ml of acetonitrile in portions, and the system gradually returns to a non-viscous state after proper adjustment. The reaction was continued for 4h (TLC reaction was complete). Stop heating, cool down in an ice-water bath, add 1.4L of water, stir and crystallize for 30min. Suction filtration, the filter cake was washed with 700ml of water, and air-dried at 60°C to obtain the product with a yield of 96%;
[0051] 2. Preparation of DXB-ABC
[0052]Add 100g of DXB-AC and 2L of acetonitrile into the three-necked flask, stir, and the system becomes a white turbid liquid. A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com