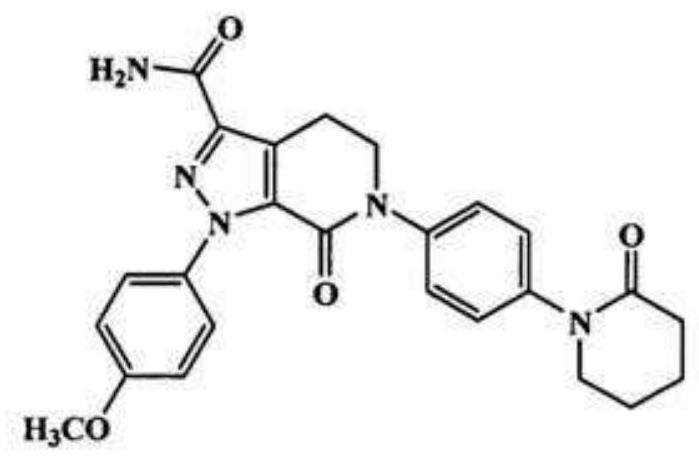
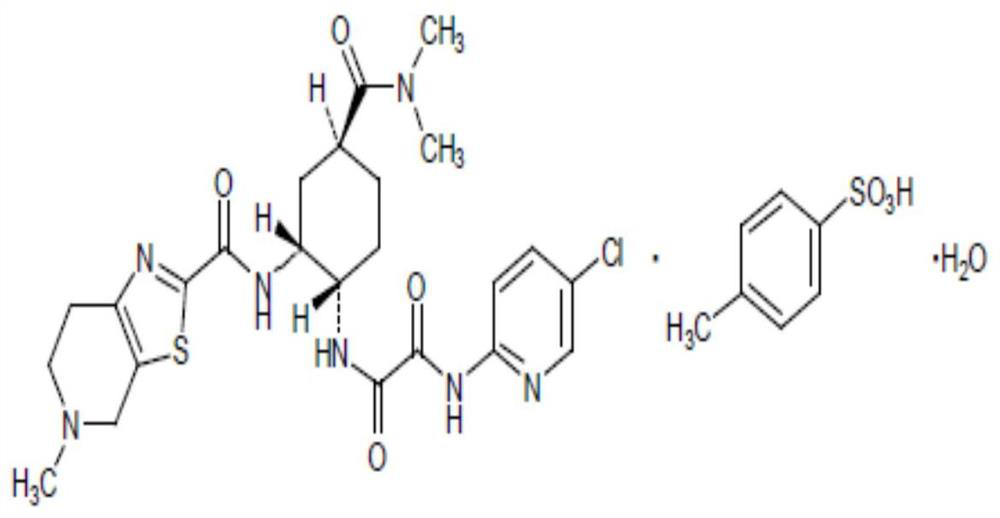
Compound sustained-release preparation containing apixaban and preparation method thereof
A sustained-release preparation, the technology of apixaban, which is applied in the field of preparation of edoxaban-apixaban compound sustained-release tablets, can solve the problems of slow drug release rate, unstable blood drug solubility, etc. Compliance, good therapeutic effect, and safety-improving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
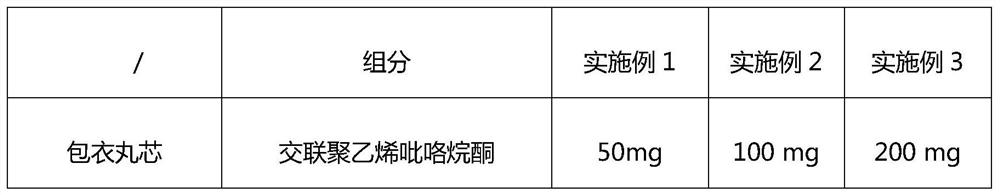
Examples
Embodiment 4
[0049] Embodiment 4 release evaluation
[0050] Determination of release rate: sample 10 samples of edoxaban and apixaban compound sustained-release tablets prepared in each embodiment, accelerate for 6 months under the conditions of 40°C and 75% RH, and then use the dissolution method according to the release rate determination method. The device of the first method for measuring the degree of concentration uses purified water as the solvent, and the rotating speed is 100 revolutions per minute. It is operated according to the law and measured according to the ultraviolet spectrophotometry method. The absorbance is measured at a wavelength of 237nm; Edoxaban and Apixa are taken separately Take an appropriate amount of reference substance, add the above-mentioned solvent to dissolve and quantitatively dilute to a solution of about 20ug per 1ml, measure the absorbance in the same way, calculate the release rate by the external standard method, and take the average value of all t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com