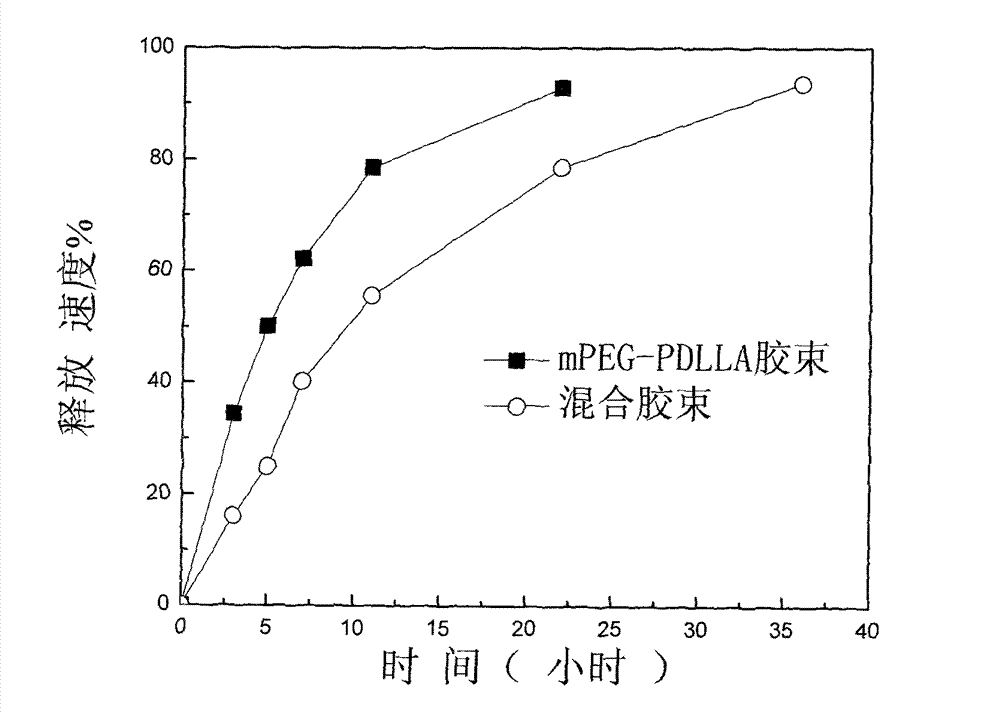
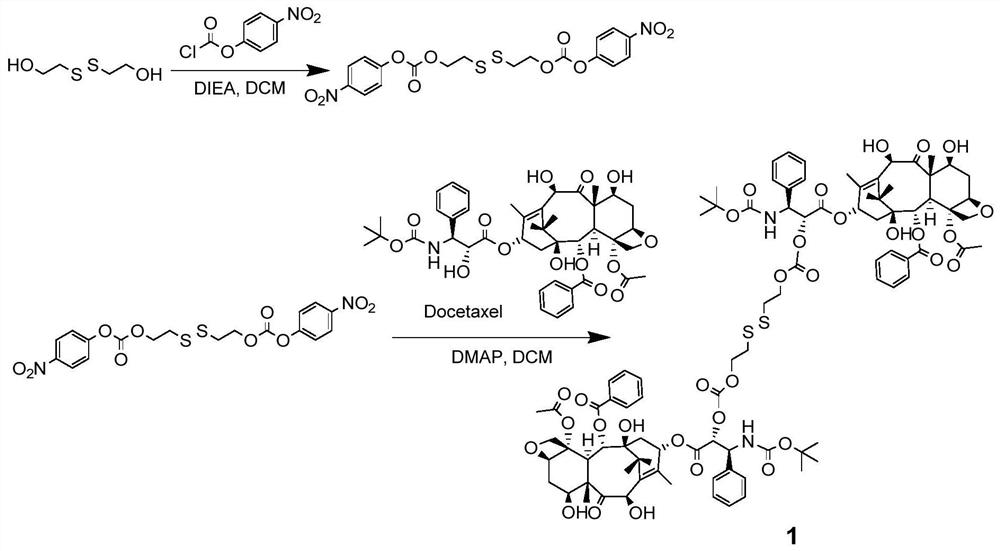
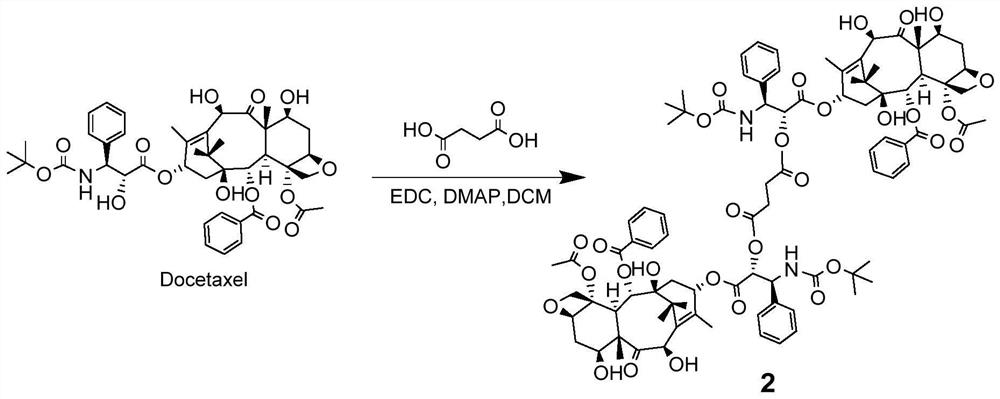
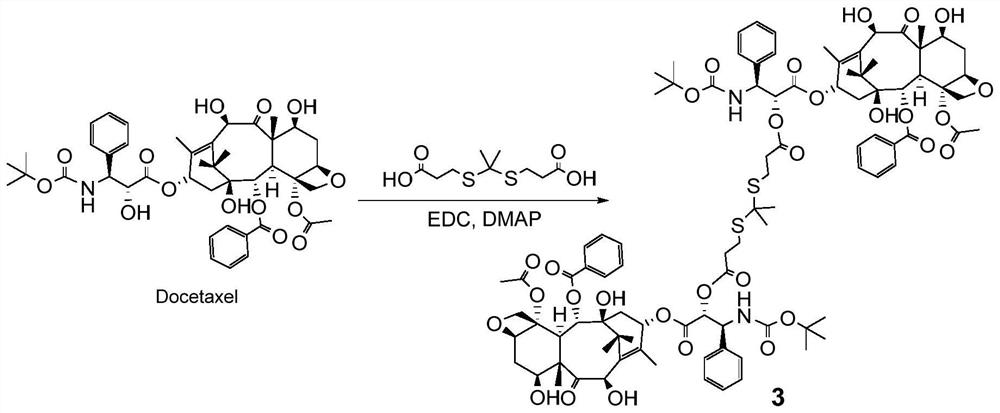
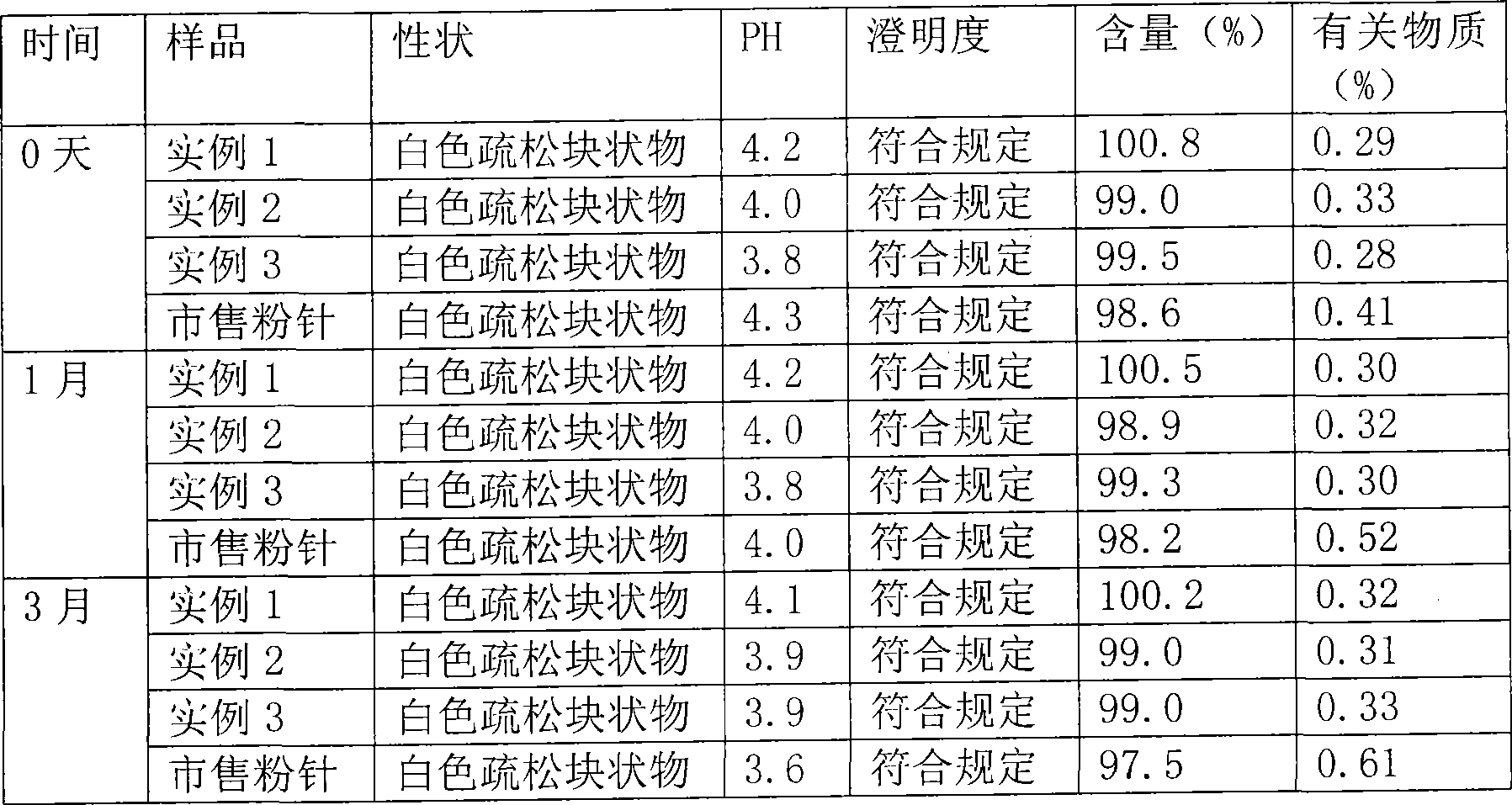
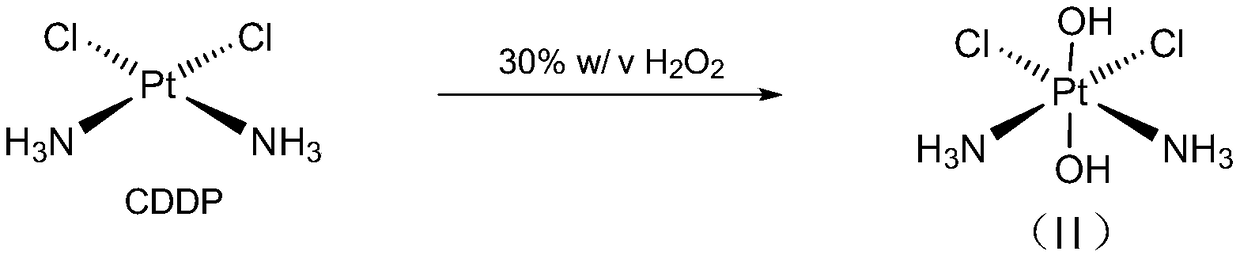
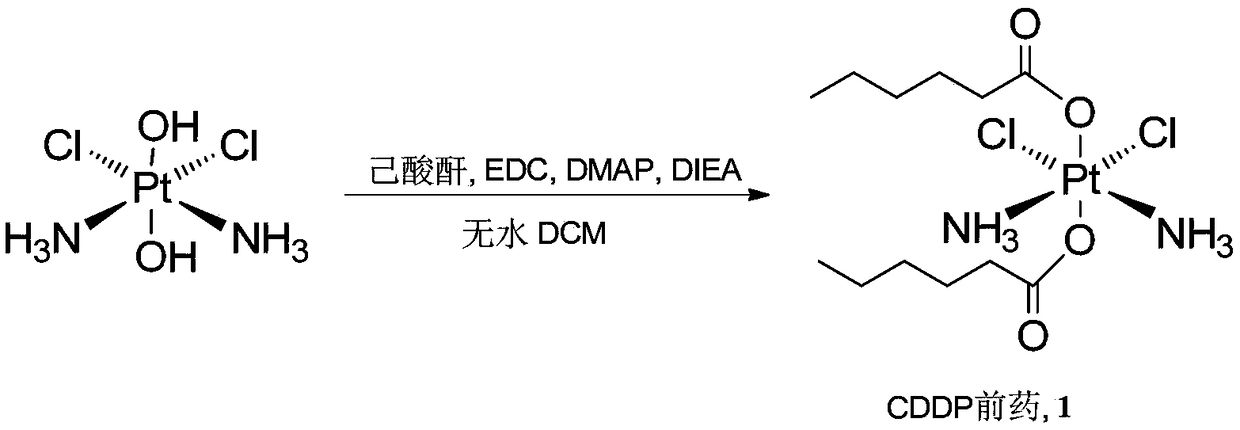
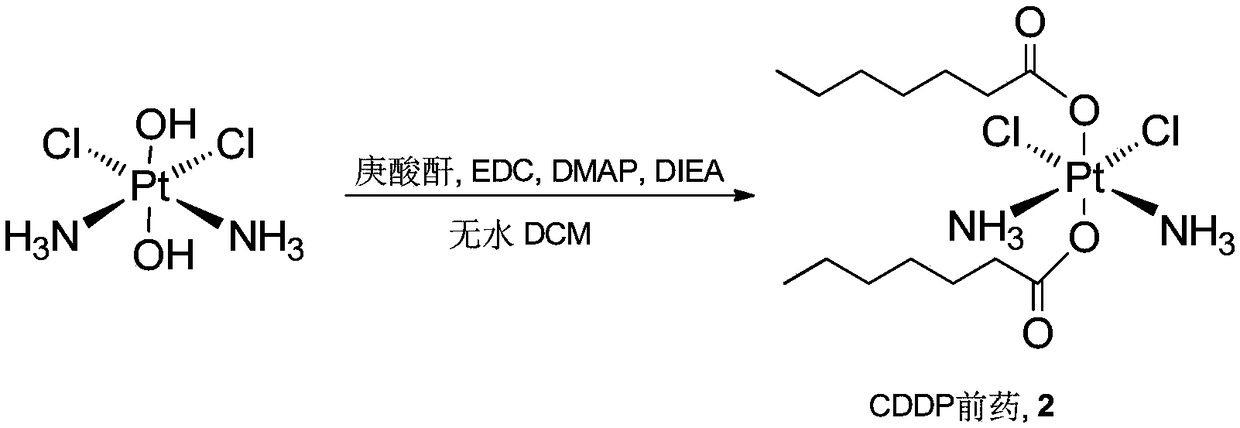
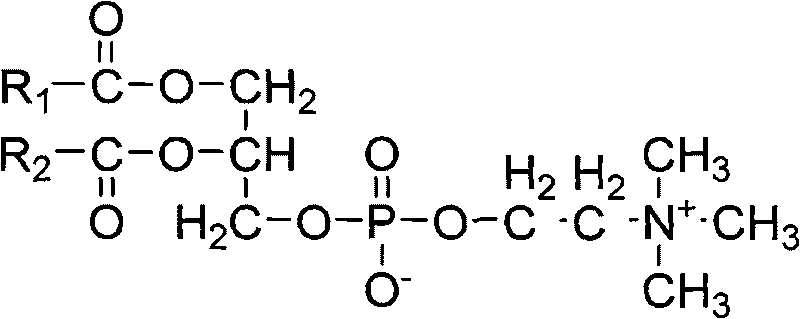
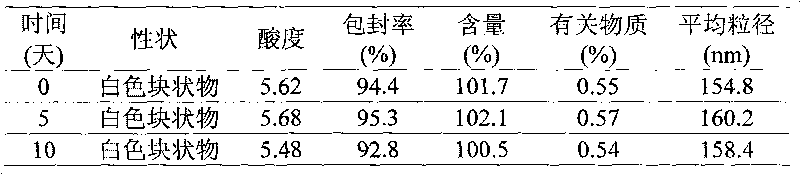
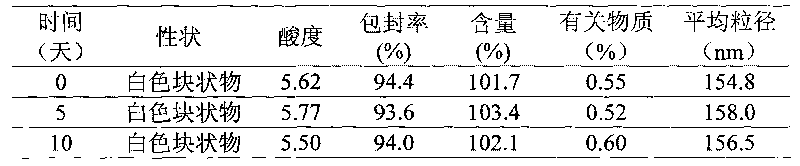
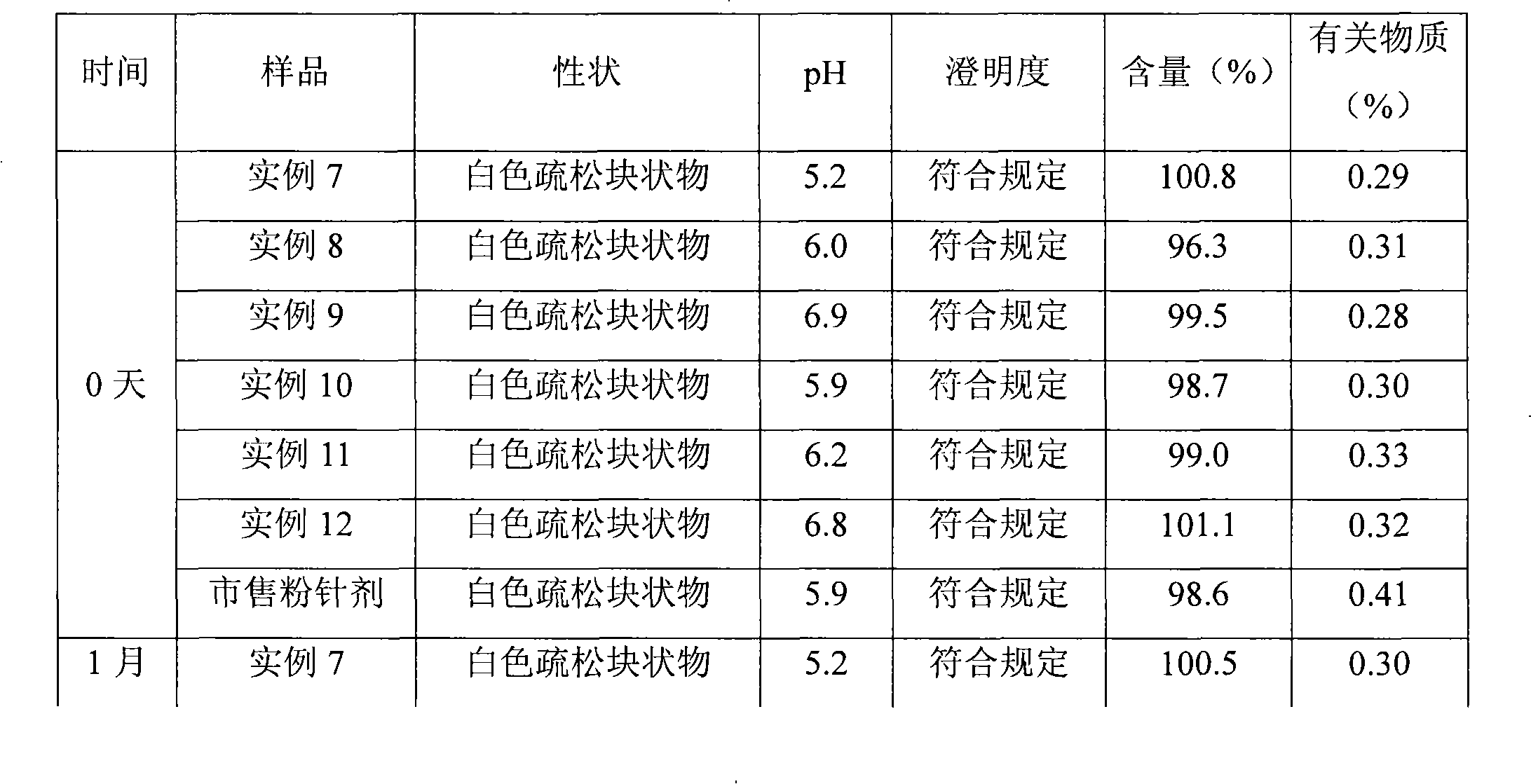
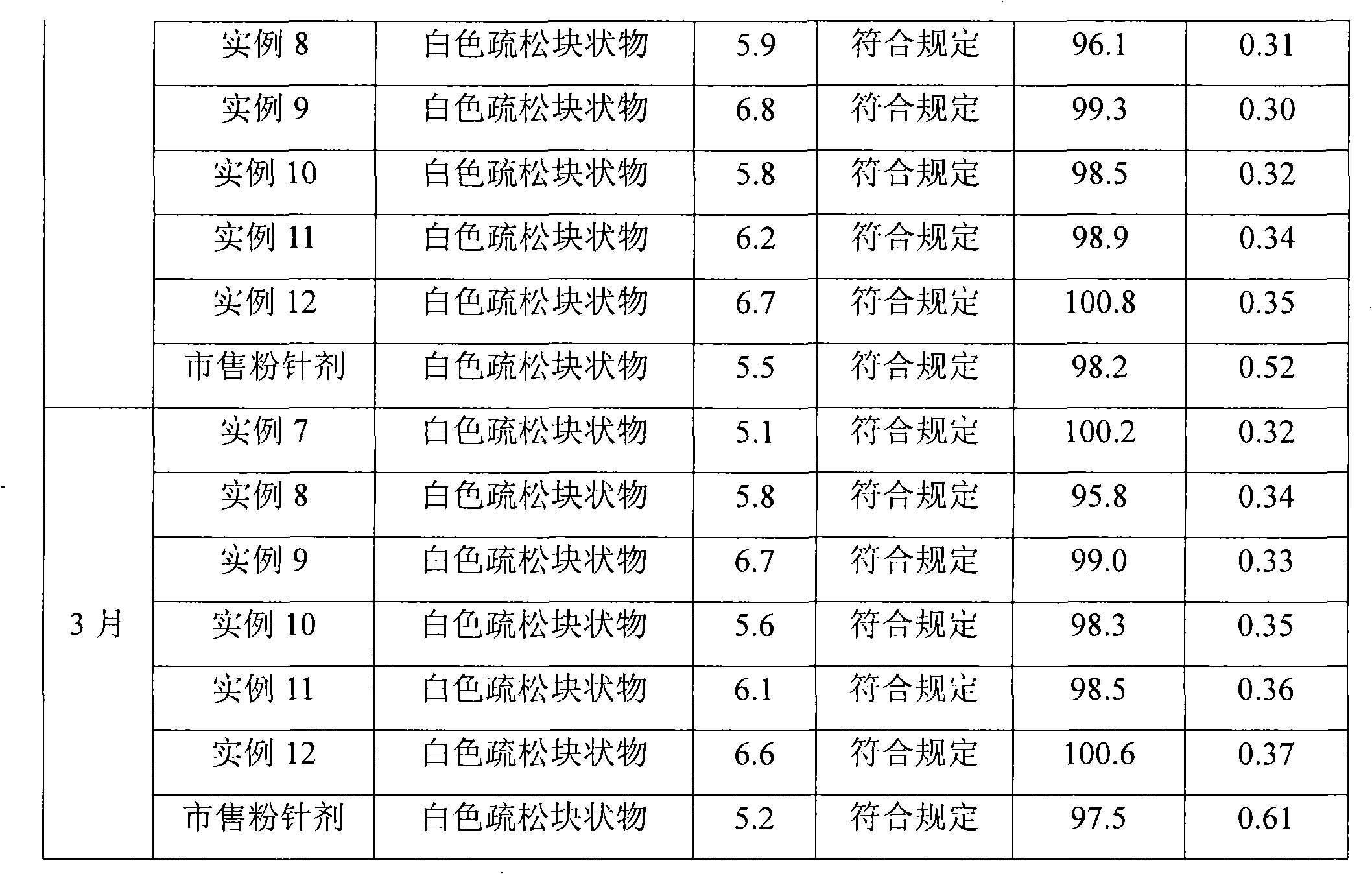
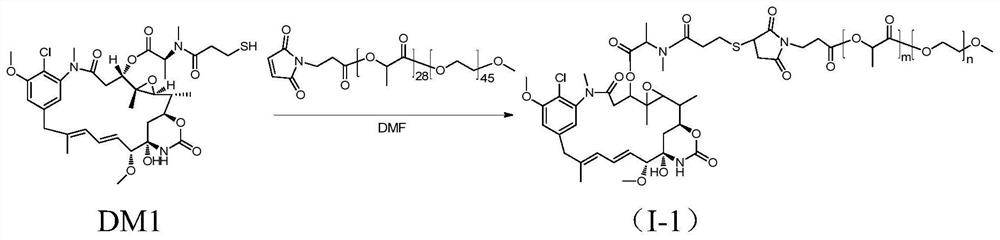
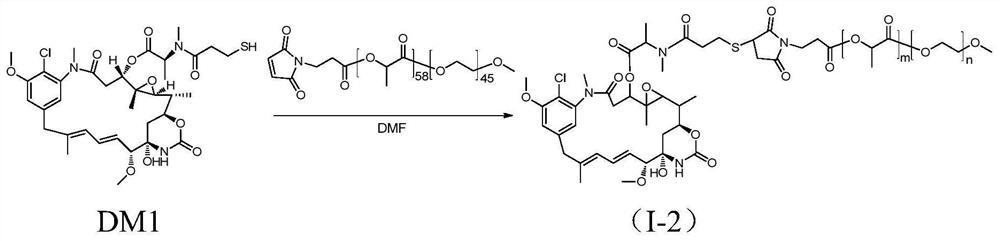
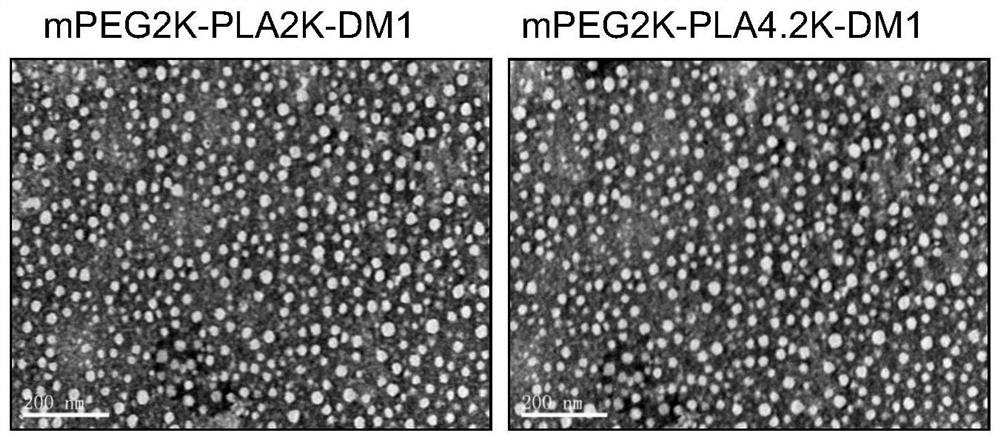
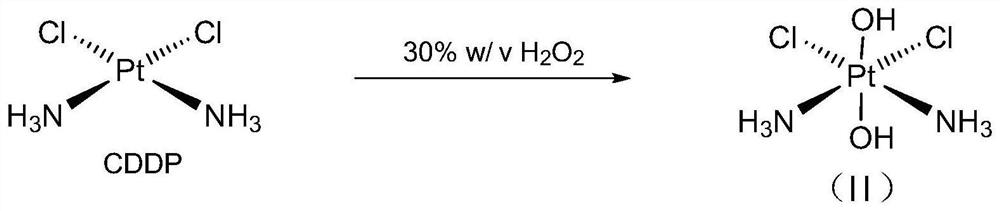
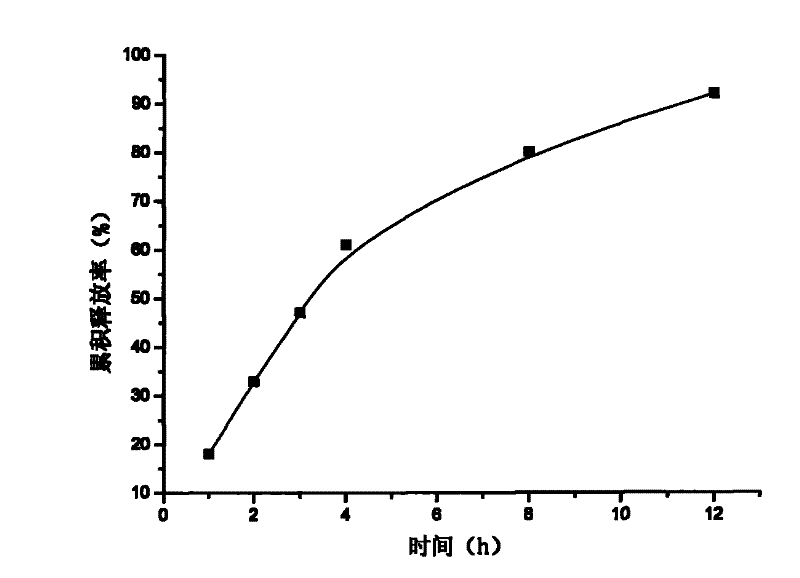
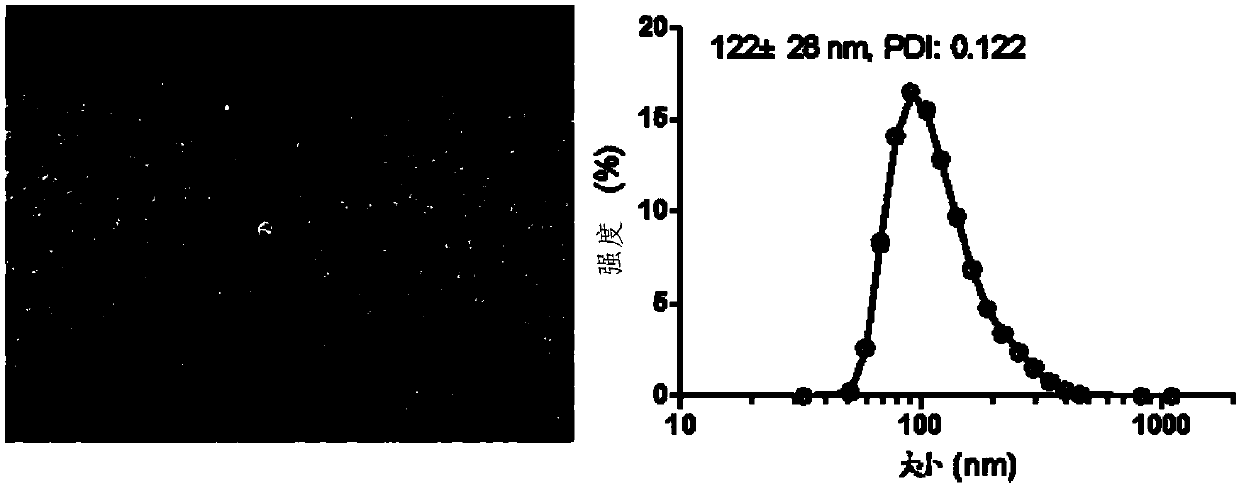
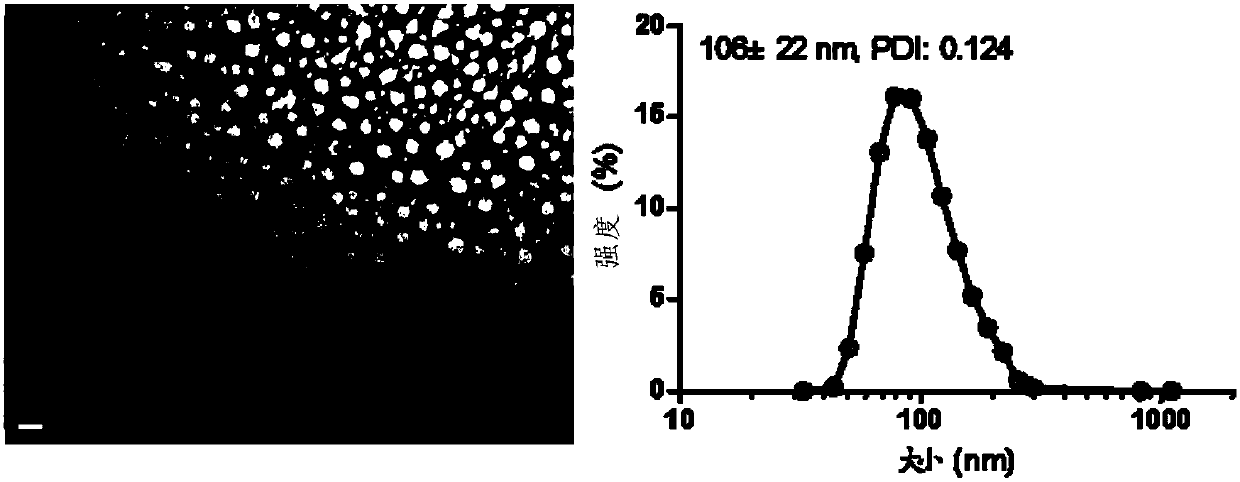
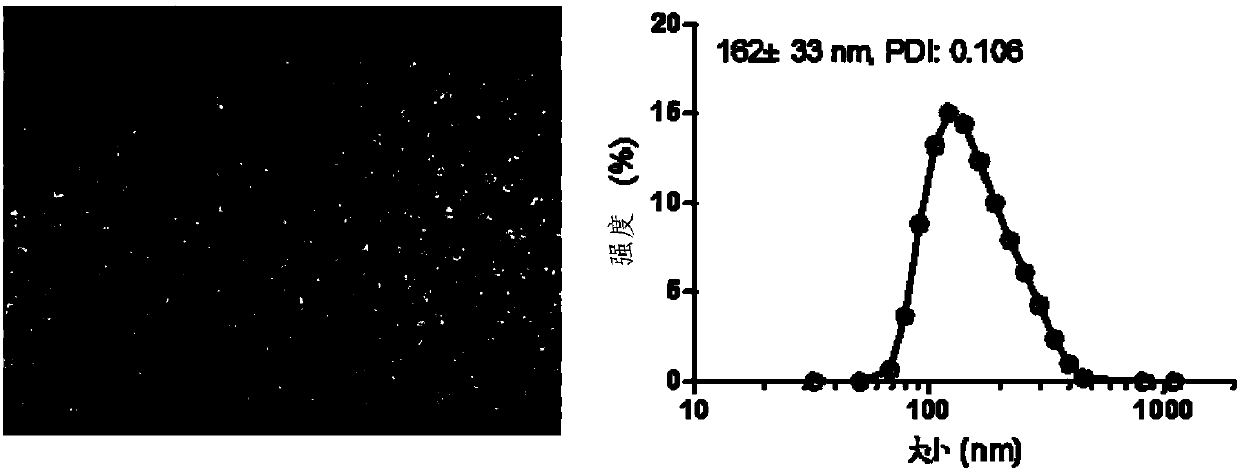
Patents
Literature
30results about How to "Meet the requirements of clinical medication" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Highly effective method for producing adenovirus
InactiveCN101235365AIncrease productionScale upGenetic material ingredientsViruses/bacteriophagesMicrobiologyBioreactor
The invention relates to a method for producing adenovirus hominis, the method comprises the following steps: inoculating host cell, leading cells to grow in culture medium, replacing culture solution in a bioreactor, utilizing adenovirus hominis to infect the host cell, breeding virus, gathering virus suspension liquid and concentrating through monitoring virus concentration in the bioreactor, and the method also comprises utilizing chromatography to separate adenovirus hominis. The producing method can produce adenovirus hominis which is in accordance with the requirement of clinical medication in large scale. The method has the advantages of high yield, large scale and low production cost, which is suitable for industrial production.
Owner:TSINGHUA YUANXING BIO PHARM SCI & TECH
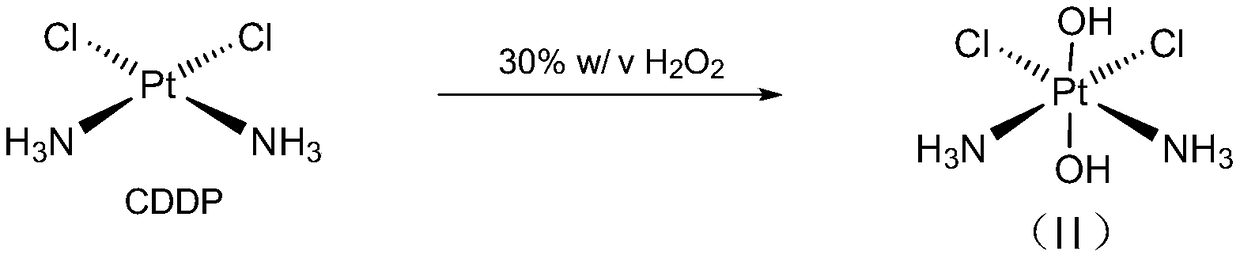
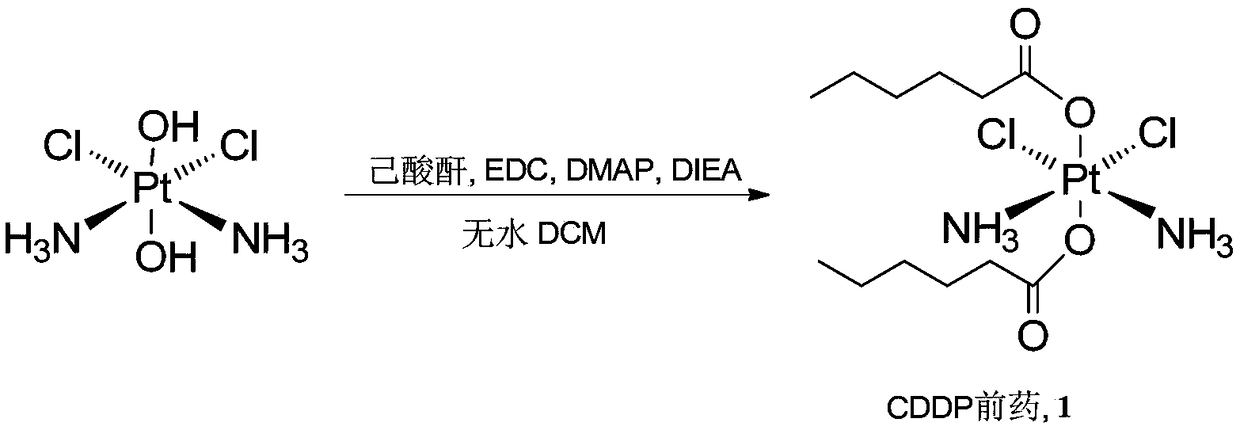
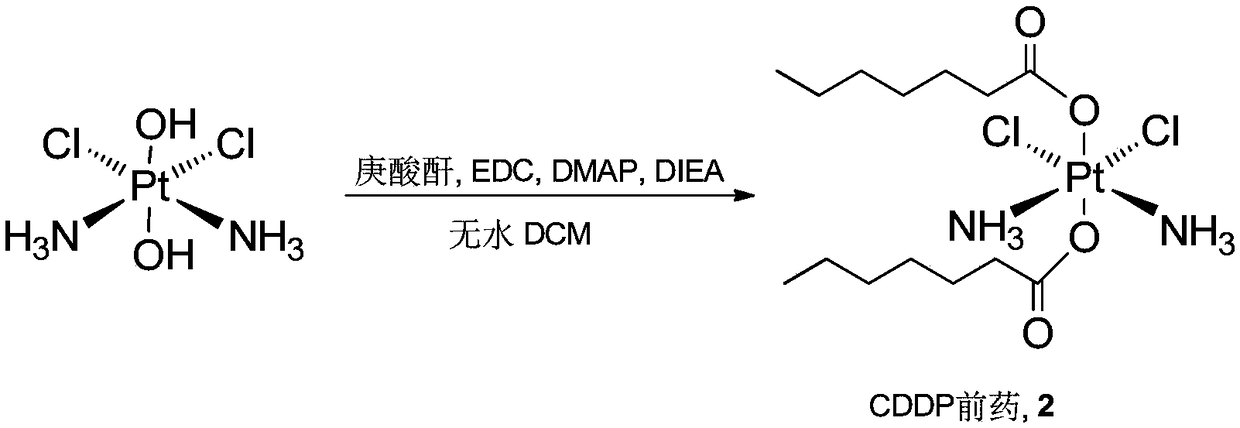
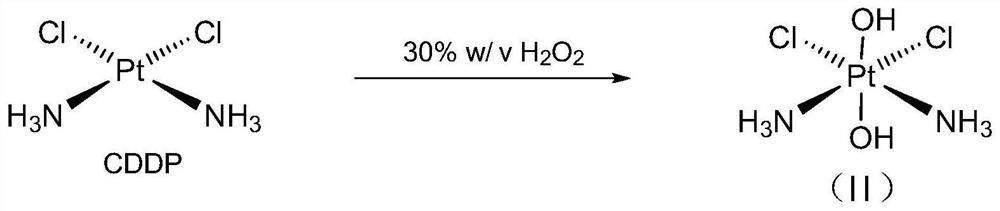
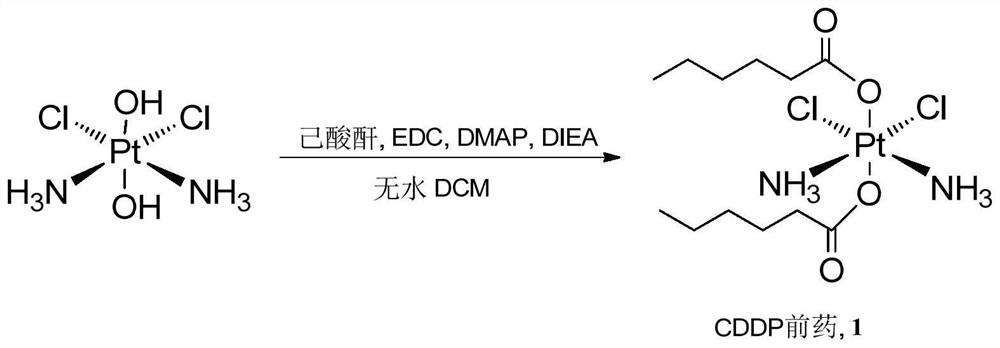
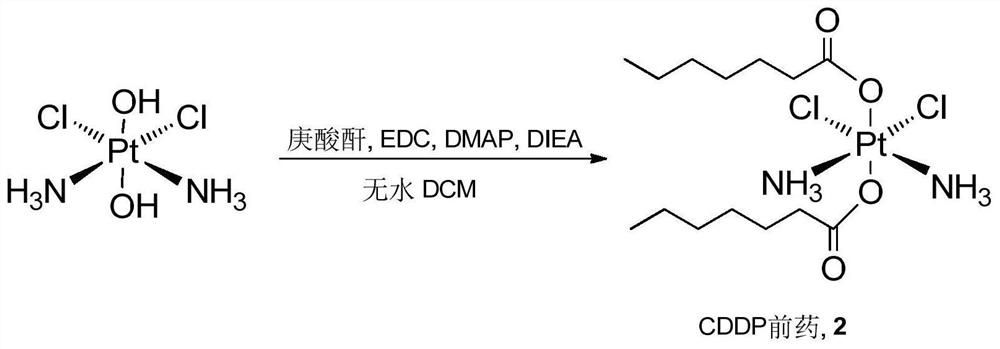
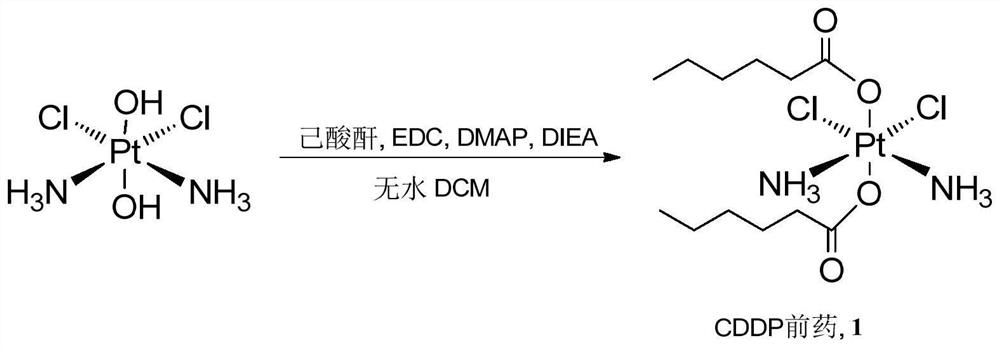
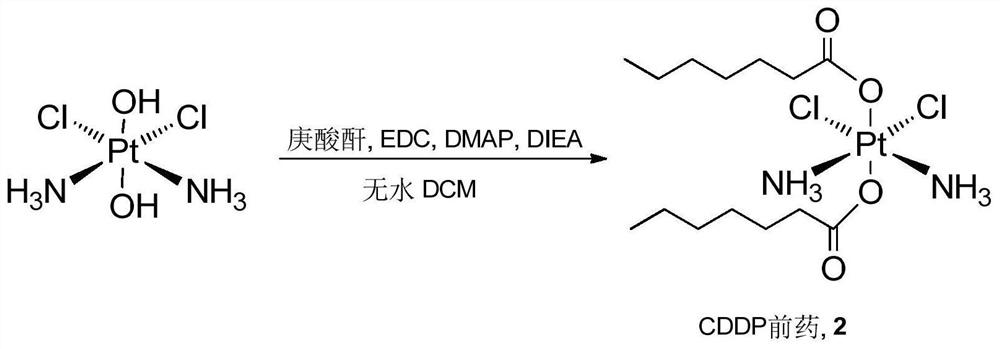
Cis-dammine dichloroplatinum prodrug, preparation method and application
InactiveCN109021026ABiologically activeExcellent ability to kill tumor cellsHeavy metal active ingredientsPlatinum organic compoundsStructural formulaWilms' tumor
The invention discloses a cis-dammine dichloroplatinum (CDDP) prodrug, a preparation method and application. The structural formula of the CDDP prodrug is shown as formula (I), and is generated by theesterification reaction of activated dihydroxy cisplatin with hydrophobic molecules. Characterization of the nano-preparation by dynamic light scattering and transmission electron microscopy indicates that the nanoparticles involved in the invention are uniformly distributed and are at about 30nm. In vitro cytotoxicity experiments show that the nano-drug can significantly inhibit the proliferation of tumor cells (A549 and LoVo). In vivo experiments show that compared with CDDP injections, on the basis of reducing the systemic toxicity, the nano-drug has the effect of inhibiting the non-smallcell lung cancer A549 subcutaneous tumor, and has good market prospects and clinical application value.
Owner:ZHEJIANG UNIV
Method for preparing matrine slow-release tablet by applying attapulgite
InactiveCN101716158AIncrease added valueUniform colorOrganic active ingredientsAntipyreticMatrineDesorption
The invention discloses a method for preparing matrine slow-release tablets by applying attapulgite, which comprises the following steps of: dissolving matrine by using hydrochloric acid with the mass concentration of 0.1 percent to obtain 2-5mg / ml of matrine solution; adsorbing the matrine solution through modified attapulgite for 4-6 hours according to the proportion by weight of 1:2; filtering and drying to obtain the attapulgite loading the matrine; and adding 2 percent by weight of talcum powder into the attapulgite loading the matrine, granulating by a dry method, and tabletting to obtain the matrine slow-release tablets. A modifying method of the attapulgite comprises the following steps of: weighing 20g of attapulgite, placing the attapulgite into 1000ml of aqueous solution, stirring for 12 hours, settling, removing upper water, adding purified water, stirring for 12 hours, settling, taking an intermediate fine particle layer, adding concentrated hydrochloric acid, settling for 24 hours, filtering through suction, washing with water to be neutral, drying at the temperature of 105DEG C, finely grinding and screening with a 120-mesh screen to obtain acid modified attapulgite. The invention adopts the principle of adsorption separation and selects the modified attapulgite which can be taken as an adsorbent for adsorbing the matrine, the matrine slow-release tablets are slowly released under the desorption action of a body fluid, operation steps are simple, and the production period of traditional Chinese medicine preparations is shortened.
Owner:HUAIYIN INSTITUTE OF TECHNOLOGY
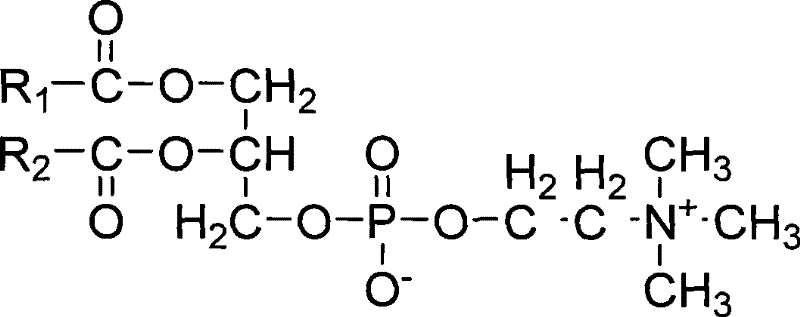
Drug-loaded mixed micelle
InactiveCN102961322AImprove solubilityHigh encapsulation efficiencyOrganic active ingredientsPharmaceutical delivery mechanismPolyoxyethylene castor oilPolyester
The invention discloses a drug-loaded mixed micelle comprising taxane medicaments, amphiphilic chitosan derivatives and polyethylene glycol polyester block copolymer. The invention further discloses a preparation method of the drug-loaded mixed micelle. The product does not contain polyoxyethylene castor oil and ethanol, reduces adverse drug reactions, increases security of drug clinic application; by adding the amphiphilic chitosan derivatives and the polyethylene glycol polyester block copolymer, the stability of the preparation is enhanced and drug loading and drug efficacy are increased; and the preparation process is simple and controllable, and the production can be expanded easily.
Owner:HANGZHOU PUSH KANG BIOTECH CO LTD
Taxane prodrug, preparation method and application thereof
PendingCN112250647AIncreased Tolerated DoseReduce toxicity in vivoOrganic active ingredientsOrganic chemistryCabazitaxelDocetaxel
The invention discloses a taxane prodrug, which has a structure of Y1-R-Y2, wherein the Y1 and the Y2 are docetaxel or cabazitaxel, R comprises a specific connecting bond for environmental response intumor cells, and the taxane prodrug is generated by carrying out substitution or condensation reaction on a taxane drug and a tumor microenvironment-responsive connecting bond. According to the invention, the prodrug has good anti-tumor activity, can directly release active ingredients in vivo in a hydrolysis or oxidation mode, and can avoid in vivo toxicity caused by direct injection of taxane drugs; the prodrug disclosed by the invention not only has good solubility in water, but also can be self-emulsified in water to form nanoparticles; and the prodrug can be obtained through a single-step reaction method, the yield is high, the preparation cost is low, the stability is high, the safety is good, the requirements of clinical medication are met, the requirements of large-scale industrial production are met, and the prodrug has good market prospects and clinical application value.
Owner:ZHEJIANG UNIV
Meclofenoxate hydrochloride preparation freeze-drying technique and preparation method thereof
InactiveCN101455646AReduce hydrolysis reactionImprove bioavailabilityPowder deliveryOrganic active ingredientsMANNITOL/SORBITOLFreeze-drying
The present invention relates to a technique of lyophilized preparation of meclofenxate hydrochloride and a preparing method thereof. According to the invention, mannitol with effective dose of medicament is added with injection water and is dissolved. The meclofenxate hydrochloride with effective dose of medicament is added and mixed to uniform. The pH value is adjusted to 3-5. The obtained preparing is adsorbed with 0.01% of active carbon and is filtered. Then free drying is executed for preparing the lyophilized preparation. The meclofenxate hydrochloride in the invention is enveloped by macromolecule material and greatly reduces the hydrolytic reaction of meclofenxate hydrochloride. Therefore the lyophilized preparation of meclofenxate hydrochloride prepared by the invention has enough stability in water and can totally satisfy the requirement of clinical medicine taking. Furthermore the lyophilized preparation of meclofenxate hydrochloride prepared by the invention can be stably and slowly released. The bioavailability of meclofenxate hydrochloride is increased and transparency after re-dissolving is excellent.
Owner:朗美药业(武汉)有限公司
Cis-platinum nano pharmaceutical preparation, preparation method and application
ActiveCN108836937AIncreased Tolerated DoseReduce systemic toxicityPlatinum organic compoundsPharmaceutical non-active ingredientsStructural formulaPt element
The invention discloses a tetravalent platinum pharmaceutical preparation, and a preparation method and application thereof. The tetravalent platinum pharmaceutical preparation comprises a prodrug andan amphipathic high molecular material. The prodrug has a structural formula (I). Dynamic light scattering and a transmission electron microscopy show that nanoparticles are uniformly distributed andare about 40-60 nm; an in vitro cytotoxicity test shows that the nanoparticles coated with platinum prodrug can inhibit proliferation of tumor cells (MDA-MB-468 and HT-29) obviously. An in vivo experiment shows that compared with a cis-platinum injection liquid, the cis-platinum nano pharmaceutical preparation has an effect of inhibiting subcutaneous tumor MDA-MB-468 on the basis of reducing thesystemic toxicity and has good market prospect and clinical application value.
Owner:ZHEJIANG UNIV
A kind of exosome drug delivery system and its preparation method and application
ActiveCN113292608BProtect completenessHigh drug loadingInorganic active ingredientsOrganic chemistry methodsPhospholipidDrug loading dose
The invention discloses an exosome drug delivery system and its preparation method and application, and provides an active drug loading system that uses phospholipid compounds to load cisplatin prodrugs on exosomes, so as to solve the problem of membrane rupture caused by exosome drug loading strategy and low drug loading; the present invention prepares cisplatin prodrugs in advance, prepares them into liposomes, and then mixes them in exosomes to realize active drug loading, utilizing the fusion of phospholipid compounds and exosome membranes It protects the integrity of the exosome membrane and increases the drug loading capacity of the exosome anticancer drugs.
Owner:天津外泌体科技有限公司
Docetaxel nano-particle composition
InactiveCN101732232AHigh encapsulation efficiencyUniform size distributionPowder deliveryOrganic active ingredientsCholesterolDocetaxel
The invention relates to a docetaxel nano-particle composition for injection administration and a preparation method thereof. The docetaxel nano-particle composition contains neutral phospholipid and docetaxel, and does not contain negative phospholipid and cholesterol.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Meclofenoxate hydrochloride microcapsule and method for preparing injection thereof
InactiveCN101278924AReduce hydrolysis reactionSlow releaseOrganic active ingredientsNervous disorderChemistryMeclofenoxate Hydrochloride
The invention provides a meclofenoxate hydrochloride microcapsule for injection and a production method thereof. The meclofenoxate hydrochloride microcapsule for the injection is composed of the meclofenoxate hydrochloride and adjuvant, which is characterized in that the adjuvant contains gelatin, dextran and emulsifier. The invention also provides the production method of a meclofenoxate hydrochloride freeze-dry powder and injection. As the meclofenoxate hydrochloride microcapsule with high stability in water is used, the meclofenoxate hydrochloride is not hydrolyzed when redissolving; the clarity is good after redissolving; thereby a product in the invention has the advantages of good stability and good quality, which is good for storing the product for a long time.
Owner:HAINAN LINGKANG PHARMA CO LTD
Recombinant leukocyte inhibitory factor and leech peptide chimeric protein freeze-dried preparation for injection and preparation method thereof
PendingCN113368063AReduce degradationReduce aggregationPowder deliveryPeptide/protein ingredientsWhite blood cellChimera Protein
The invention belongs to the technical field of protein and polypeptide drugs, and particularly relates to a recombinant leukocyte inhibitory factor and leech peptide chimeric protein freeze-dried preparation and a preparation method thereof. The lyophilized powder for injection comprises the recombinant leukocyte inhibitory factor and leech peptide chimeric protein, an excipient, a cryoprotectant, and a buffer system. By researching different excipients, cryoprotectants, buffer systems and freeze-drying curves, the recombinant leukocyte inhibitory factor and leech peptide chimeric protein freeze-drying preparation for injection is provided, which is good in appearance, good in resolubility, high in activity, less in impurity, low in side effect, high in safety and stable in quality. The problems of unstable protein, easy aggregation and denaturation, reduced activity and the like are solved. The preparation is convenient to use, quick to absorb and convenient for storage and transport.
Owner:LUNAN PHARMA GROUP CORPORATION
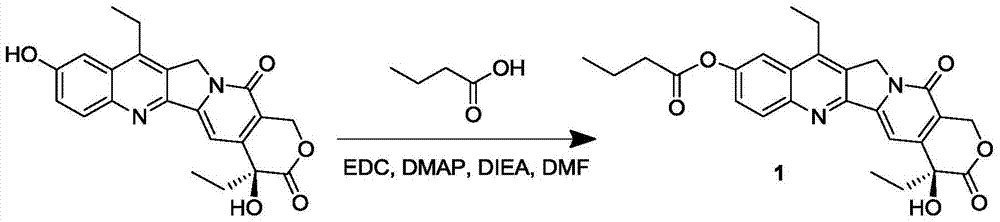
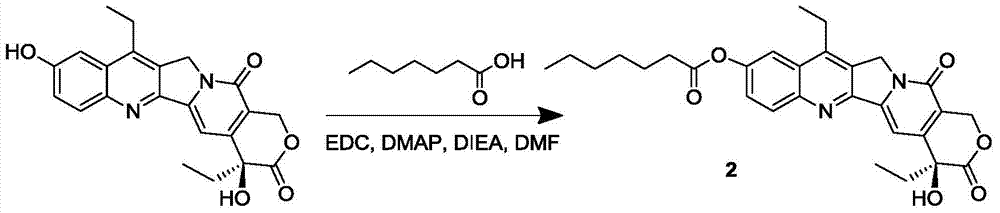
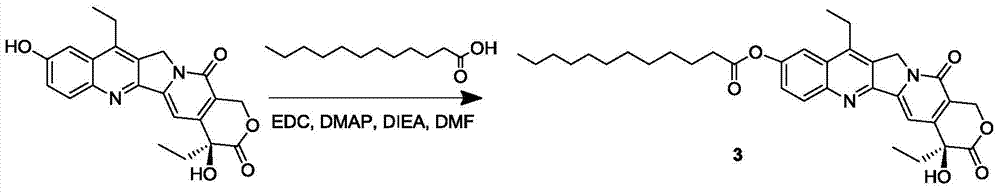
7-ethyl-10-hydroxycamptothecin prodrug and its preparation method and application
InactiveCN105315294BGood antitumor activityImprove bioavailabilityOrganic active ingredientsOrganic chemistrySolubility7-ethyl-10-hydroxycamptothecin
The invention discloses a 7-ethyl-10-hydroxycamptothecine drug precursor, a preparation method and an application thereof. A structure formula of the drug precursor is represented as the formula I or II. The drug precursor is prepared through an esterification reaction between a C-10 hydroxyl group or a C-20 hydroxyl group of 7-ethyl-10-hydroxycamptothecine and a hydrophobic molecule. The drug precursor has excellent anti-tumor activity and can directly release active components in vivo in a hydrolysis manner without catalytic hydrolysis of carboxylesterase, thereby achieving a high bioavailability. The drug precursor not only has excellent solubility in water but also has great solubility in amphipathic surfactants, such as tween-80 and the like, wherein the solubility can reach more than 30 mg / ml, and a high stability is achieved even that the drug precursor is diluted in water. The drug precursor can be prepared just through a one-step esterification method, is high in yield and low in preparation cost, is high in stability and good in safety, satisfies requirements in clinical medication and in large-scale industrial production, and has excellent market prospect and clinical application value.
Owner:王杭祥
Cisplatin prodrug, preparation method and application
InactiveCN109021026BBiologically activeExcellent ability to kill tumor cellsHeavy metal active ingredientsPlatinum organic compoundsStructural formulaDrug precursors
The invention discloses a cis-dammine dichloroplatinum (CDDP) prodrug, a preparation method and application. The structural formula of the CDDP prodrug is shown as formula (I), and is generated by theesterification reaction of activated dihydroxy cisplatin with hydrophobic molecules. Characterization of the nano-preparation by dynamic light scattering and transmission electron microscopy indicates that the nanoparticles involved in the invention are uniformly distributed and are at about 30nm. In vitro cytotoxicity experiments show that the nano-drug can significantly inhibit the proliferation of tumor cells (A549 and LoVo). In vivo experiments show that compared with CDDP injections, on the basis of reducing the systemic toxicity, the nano-drug has the effect of inhibiting the non-smallcell lung cancer A549 subcutaneous tumor, and has good market prospects and clinical application value.
Owner:ZHEJIANG UNIV
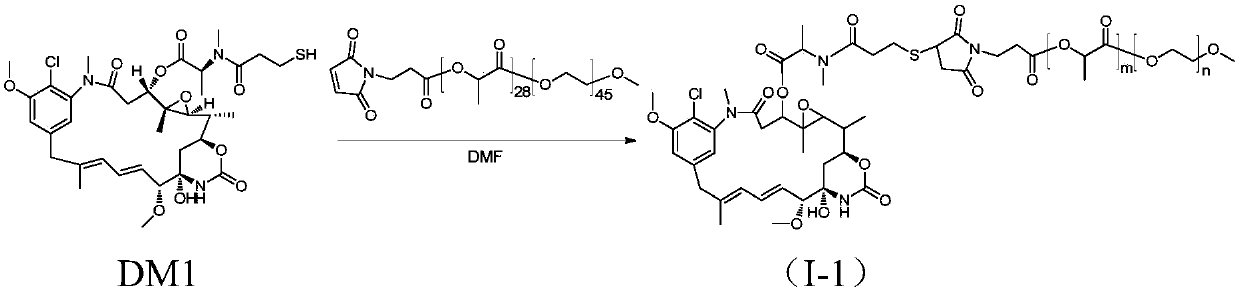
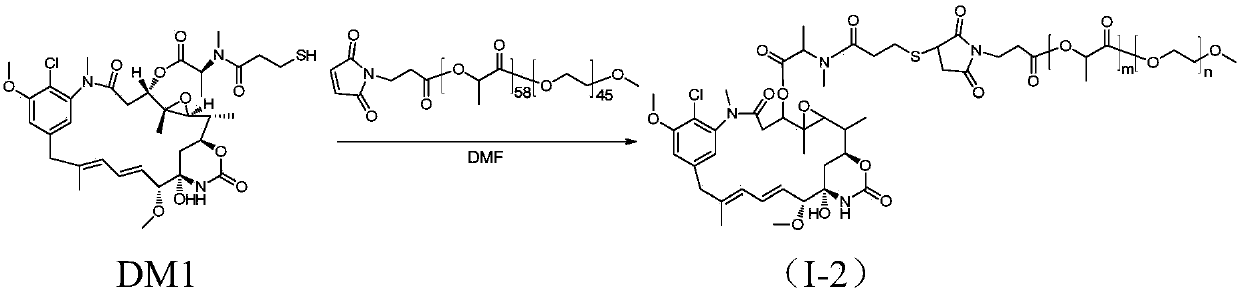
Amphiphilic copolymer-maytansine covalent drug conjugates, preparation method and application
InactiveCN107913410ALow cytotoxicitySmall toxicityOrganic active ingredientsPharmaceutical non-active ingredientsChemical reactionDrug precursors
The invention discloses amphiphilic copolymer-maytansine covalent drug conjugates, a preparation method and an application thereof. The structure of the amphiphilic copolymer-maytansine covalent drugconjugates is R-X-may, wherein X is a connection piece, R is derived from amphiphilic polymers and may is a maytansine base. A drug precursor and nano-micelle loaded with an anti-tumor drug can be obtained through simple chemical reactions, and release of a maytansine drug in blood can be significantly reduced, so that the drug conjugates are expected to greatly reduce damage to normal tissue andorgans. The drug conjugates are low in preparation cost, high in stability and good in safety, meet the clinical medication requirement, meet large-scale industrial production and have good market prospects and clinical application value.
Owner:ZHEJIANG UNIV
Preparation process of galangal and rhizoma cyperi liquid capsule
InactiveCN101926949BMaximum extractionAdequate doseDigestive systemCapsule deliveryVegetable oilExtraction methods
The invention relates to a preparation process of a galangal and rhizoma cyperi liquid capsule, which comprises the following processing steps: (1) respectively pulverizing galangal and rhizoma cyperi based on the weight ratio of 1:1 into coarse powder; (2) extracting the galangal by a carbon dioxide supercritical extraction method to obtain the extract of the galangal; (3) extracting the rhizomacyperi by a carbon dioxide supercritical extraction method to obtain the extract of the rhizoma cyperi; (4) mixing the galangal extract and the rhizoma cyperi extract and dehydrating to obtain the galangal and rhizoma cyperi extract; (5) adding vegetable oil and tween 80 to the galangal and rhizoma cyperi extract and mixing evenly to obtain galangal and rhizoma cyperi liquid; and (6) filling the galangal and rhizoma cyperi liquid and sealing to obtain the galangal and rhizoma cyperi liquid capsule. The invention respectively adopts different extraction conditions for carrying out carbon dioxide supercritical extraction on the effective components of the galangal and the rhizoma cyperi, reserves the effective components in the raw materials and improves the extraction rate, and the prepared galangal and rhizoma cyperi liquid capsule has the characteristics of safety, reliability, convenient taking, small dose, convenient carrying and the like.
Owner:TIANJIN PACIFIC PHARMA
A kind of docetaxel nanoparticle composition
InactiveCN101732232BImprove stabilityImprove medication safetyOrganic active ingredientsPowder deliveryNanoparticleDocetaxel
The present invention relates to a docetaxel nanoparticle composition that can be used for injection administration and a preparation method thereof. The docetaxel nanoparticle composition of the present invention contains neutral phospholipid and docetaxel, and the composition does not contain negative phospholipid and cholesterol.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
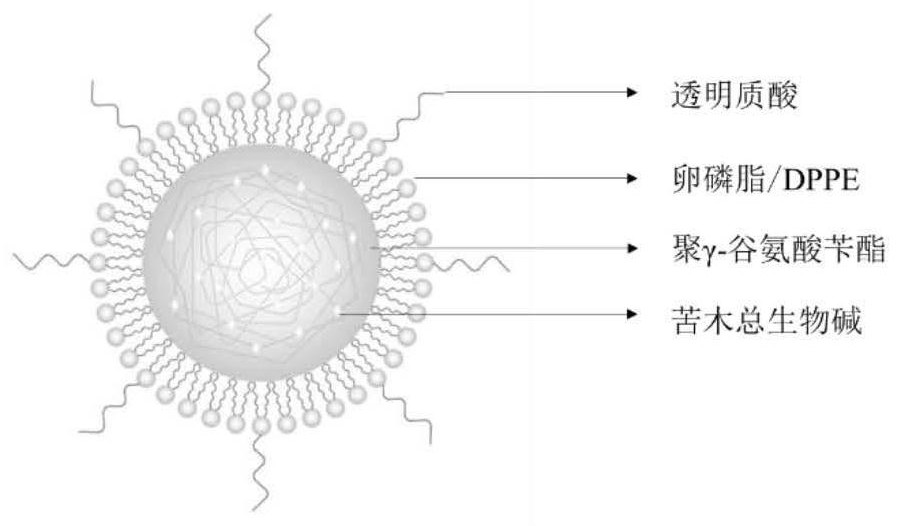
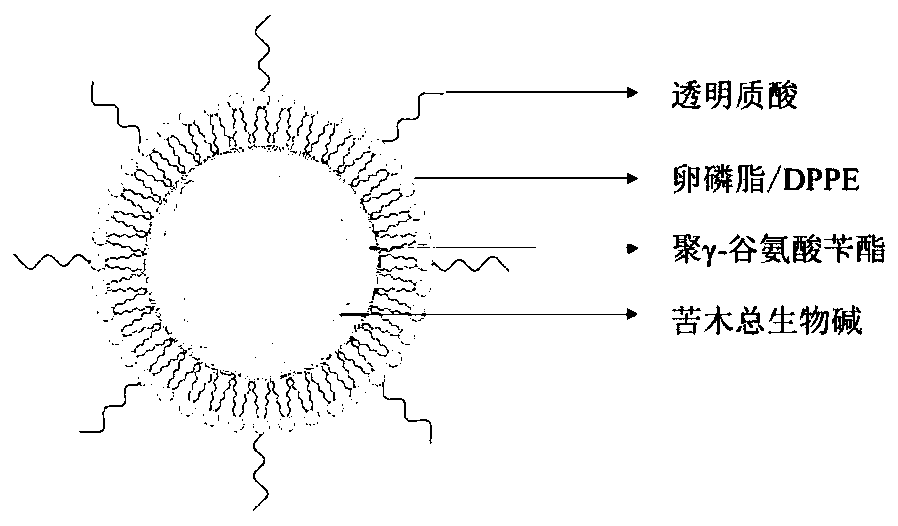
Hyaluronic acid-modified total alkaloid hybrid lipid nano-preparation and its preparation method and application
ActiveCN109718260BLow toxicityGood anti-inflammatory activityAntipyreticAnalgesicsBiotechnologyMomordica
The present invention relates to the total alkaloid hybrid lipid nano-preparation of bitterwood, which discloses the total alkaloid hybrid lipid nano-preparation of bitterwood modified by hyaluronic acid and its preparation method and application; ‑BzPGA) as the core material, amphiphilic compounds such as soybean lecithin containing 98% phosphatidylcholine (PC) and dipalmitoylphosphatidylethanolamine (DPPE) as surfactants, hyaluronic acid (HA) or Polyethylene glycol-b-palmitic acid (PEG-b-C16) is the shell material; this carrier can be used to load the total alkaloids of Materia lanceolata. The preparation method is mature and efficient, and provides the possibility for the preparation of the complex active ingredients of the total alkaloids and other active monomer nano-preparations.
Owner:CHINA PHARM UNIV
Tigecycline composition and preparation method thereof
ActiveCN102138925BReduce generationImprove stabilityAntibacterial agentsTetracycline active ingredientsVitamin CTigecycline
The invention relates to a tigecycline composition belonging to the field of medicament preparations, in particular to a tigecycline composition suitable for injection and a preparation method thereof. The composition provided by the invention is prepared by adding one or more selected from Vitamin C or pharmaceutically acceptable salts thereof and amino acids. The tigecycline composition disclosed by the invention not only effectively restricts oxidative degradation, but also remarkably decreases the epimerization of tigecycline and improves the stability of the tigecycline.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Preparation method and application of cabazitaxel prodrug
ActiveCN106432141BIncreased Tolerated DoseReduce toxicity in vivoOrganic chemistryAntineoplastic agentsSolubilityCabazitaxel
The invention discloses a cabazitaxel prodrug as well as a preparation method and an application thereof. A structural formula of the prodrug is represented as a formula (I) and the prodrug is prepared from cabazitaxel and hydrophobic molecules through an esterification reaction. The prodrug has better antitumor activity, can directly release active components in a hydrolytic manner in vivo, and can prevent in-vivo toxicity caused by direct injection of cabazitaxel. The prodrug has better solubility in water and can form nanoparticles in water through self-emulsification; the prodrug can be obtained with a single-step esterification method, is high in yield, low in preparation cost, high in stability and good in safety, meets requirements of clinical medication and large-scale industrial production, and has good market prospect and clinical application value.
Owner:ZHEJIANG UNIV
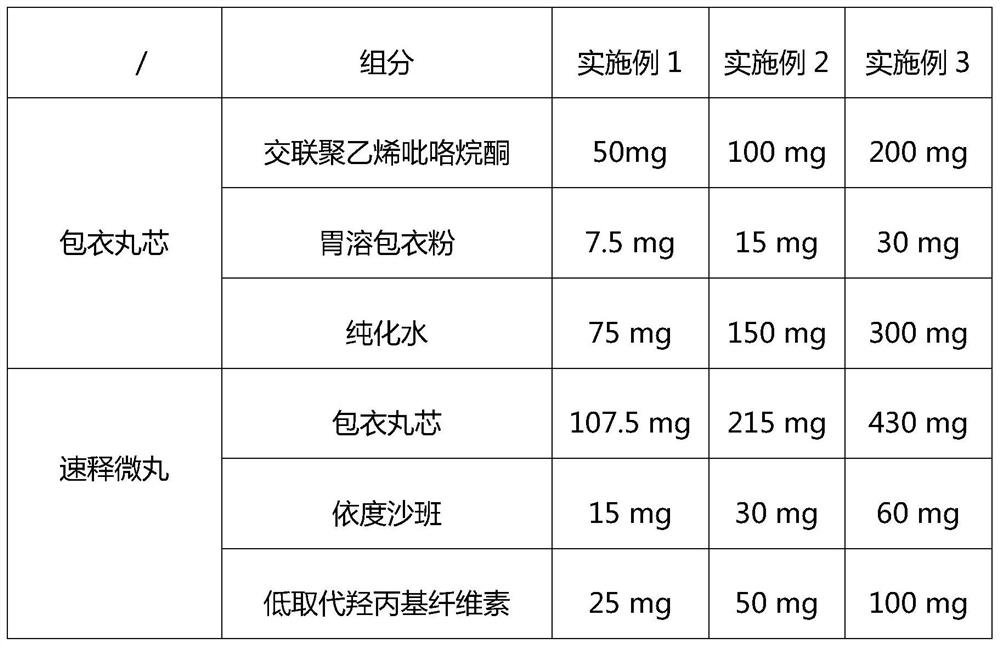
A compound slow-release preparation containing apixaban and its preparation method
ActiveCN112494489BDisintegrates quicklyReduce the number of dosesPowder deliveryOrganic active ingredientsImmediate releaseSpray coating
Owner:浙江诺得药业有限公司 +1
Cholesterol-poloxamer-cholesterol triblock copolymer and its preparation method and application
ActiveCN104163915BLow critical micelle concentrationHigh drug loadingPharmaceutical non-active ingredientsPharmaceutical active ingredientsIce waterCholesterol
The invention relates to a cholesterol-poloxamer-cholesterol triblock copolymer, a preparation method and application thereof. The triblock copolymer is obtained by taking poloxamer as the basic framework, and connecting cholesterol to both ends by carbonic ester bonds. The preparation method includes: placing poloxamer in a sealed container, adding an alkaline catalyst and an acid binding agent under a nitrogen condition, slowly adding a dichloromethane solution containing cholesteryl chloroformate dropwise, conducting stirring mixing in ice-water bath for 5-30min, then placing the mixture at room temperature to react for 1-72h, after the reaction, at the end of the reaction, reducing the pressure and removing the solvent so as to obtain a crude product; adding a proper amount of distilled water to the crude product, performing extraction with dichloromethane three times, then conducting washing three times with ice water, saturated sodium chloride and 100mM hydrochloric acid in order, and carrying out precipitation by ice ether to obtain a white wax matter; and subjecting the white wax matter to repeated precipitation refining by ice ether, thus obtaining the triblock copolymer. And the triblock copolymer has the advantages of low critical micelle concentration, large drug loading capacity, good dilution stability, simple synthetic process, low cost, and wide application range, etc. (structural formula).
Owner:SHENYANG PHARMA UNIVERSITY +1
Amphiphilic copolymer-maytansinoids covalently coupled drug, preparation method and application
InactiveCN107913410BLow cytotoxicitySmall toxicityOrganic active ingredientsPharmaceutical non-active ingredientsMaitansineDrug precursors
The invention discloses a preparation method and application of an amphiphilic copolymer-maytansinoid covalently coupled drug. The amphiphilic copolymer-maytansinoid covalently coupled drug structure is: R-X-may; wherein, X is a connecting segment; R is from an amphiphilic polymer; may is a maytansinoid group. The present invention can obtain the drug precursor through a simple chemical reaction, and obtain nano-micelles loaded with anti-tumor drugs, which can significantly reduce the release of maytansine drugs in the blood, thereby greatly reducing the effect on normal tissues and organs. damage. The preparation cost of the conjugated drug is low, the stability is high, and the safety is good, which meets the requirements of clinical medication and large-scale industrial production, and has good market prospects and clinical application value.
Owner:ZHEJIANG UNIV
Cisplatin nano drug preparation, preparation method and application
ActiveCN108836937BIncreased Tolerated DoseReduce systemic toxicityPlatinum organic compoundsPharmaceutical non-active ingredientsStructural formulaDrug precursors
The invention discloses a tetravalent platinum drug preparation, a preparation method and application thereof, including a drug precursor and an amphiphilic polymer material, and the structural formula of the drug precursor is as formula (I). Dynamic light scattering and transmission electron microscopy show that the nanoparticles in the present invention are uniformly distributed, about 40-60 nm; in vitro cytotoxicity experiments show that the nanoparticles coated with platinum prodrug can significantly inhibit tumor cells (MDA-MB-468 and HT- 29); in vivo experiments show that, compared with cisplatin injection, on the basis of reducing systemic toxicity, it has the effect of inhibiting subcutaneous tumor MDA-MB-468, and has good market prospects and clinical application value.
Owner:ZHEJIANG UNIV
Method for preparing matrine slow-release tablet by applying attapulgite
InactiveCN101716158BIncrease added valueUniform colorOrganic active ingredientsAntipyreticMatrineDesorption
The invention discloses a method for preparing matrine slow-release tablets by applying attapulgite, which comprises the following steps of: dissolving matrine by using hydrochloric acid with the mass concentration of 0.1 percent to obtain 2-5mg / ml of matrine solution; adsorbing the matrine solution through modified attapulgite for 4-6 hours according to the proportion by weight of 1:2; filteringand drying to obtain the attapulgite loading the matrine; and adding 2 percent by weight of talcum powder into the attapulgite loading the matrine, granulating by a dry method, and tabletting to obtain the matrine slow-release tablets. A modifying method of the attapulgite comprises the following steps of: weighing 20g of attapulgite, placing the attapulgite into 1000ml of aqueous solution, stirring for 12 hours, settling, removing upper water, adding purified water, stirring for 12 hours, settling, taking an intermediate fine particle layer, adding concentrated hydrochloric acid, settling for 24 hours, filtering through suction, washing with water to be neutral, drying at the temperature of 105DEG C, finely grinding and screening with a 120-mesh screen to obtain acid modified attapulgite. The invention adopts the principle of adsorption separation and selects the modified attapulgite which can be taken as an adsorbent for adsorbing the matrine, the matrine slow-release tablets are slowly released under the desorption action of a body fluid, operation steps are simple, and the production period of traditional Chinese medicine preparations is shortened.
Owner:HUAIYIN INSTITUTE OF TECHNOLOGY
A kind of antitumor drug conjugate, preparation method, preparation and application
InactiveCN106317067BImprove anti-tumor effectSimple stepsOrganic active ingredientsOrganic chemistryEthyl groupStructural formula
The invention discloses an anti-tumor medicine conjugate, a preparation method, nano-micelle preparation thereof and application. The structural formula of the conjugate provided by the invention is represented by the formula (I), and the conjugate is formed by connecting 7-ethyl-10-hydroxy camptothecin with a paclitaxel anti-tumor medicine through a connection bond, wherein L is the connection bond, R1 is phenyl or tert-butoxy, R2 is acetyl, H or methyl, and R3 is H or methyl. The steps are simple, the preparation cost is low, the stability is high, and the safety is high; the requirement on clinical medication is met; the anti-tumor medicine conjugate is suitable for large-scale industrial production. The invention further discloses nano-micelle consisting of the anti-tumor medicine conjugate represented by the formula (I) and an amphiphilic polymer and application of the nano-micelle in tumor resistance; an in-vitro test shows that the nano-micelle is good in tumor resisting effect and has relatively high market prospect and value.
Owner:ZHEJIANG UNIV
A kind of azacitidine freeze-dried powder for injection
ActiveCN108721222BGood lookingGood resolubilityPowder deliveryOrganic active ingredientsSodium acetateMannitol
The invention discloses freeze-dried azacitidine powder for injection. The freeze-dried azacitidine powder comprises azacitidine, mannitol and sodium acetate. Meanwhile, the invention discloses a preparation method of freeze-dried azacitidine powder injection for injection and aims to solve the problems of instability of azacitidine aqueous solution, non-uniform particle distribution, substandardsuspending uniformity, small settling volume and the like through adding sodium acetate, sieving bulk drug, controlling preparation temperature, optimizing freeze-drying curve and the like. A productproduced according to the technical scheme provided by the invention is stable in quality, good in redissolving performance, and uniform in particle distribution; the particle size of prepared suspension satisfies the requirements of clinical medication, the settling speed is low, caking does not exist after settling, and particles can disperse uniformly after being shaken gently.
Owner:LUNAN PHARMA GROUP CORPORATION
Cream contg. doxepin hydrochloride, and its prepn. method
InactiveCN1868450ASimple compositionSimple preparation processOrganic active ingredientsAerosol deliveryMonoglycerideDoxepin Hydrochloride
A cream of doxepin hydrochloride is prepared proportionally from doxepin hydrochloride, monoglyceride stearate, polyoxyethene-100 stearate, hexadecanol, vaseline, phenylmethanol, and water. Its preparing process is also disclosed.
Owner:湖北科益药业股份有限公司
Hyaluronic acid modified picrasma quassioides total alkaloid hybrid lipid nano preparation, and preparation method and applications thereof
ActiveCN109718260ALow toxicityIncreased anti-inflammatory activityAntipyreticAnalgesicsPicrasma quassioidesLecithin
Owner:CHINA PHARM UNIV
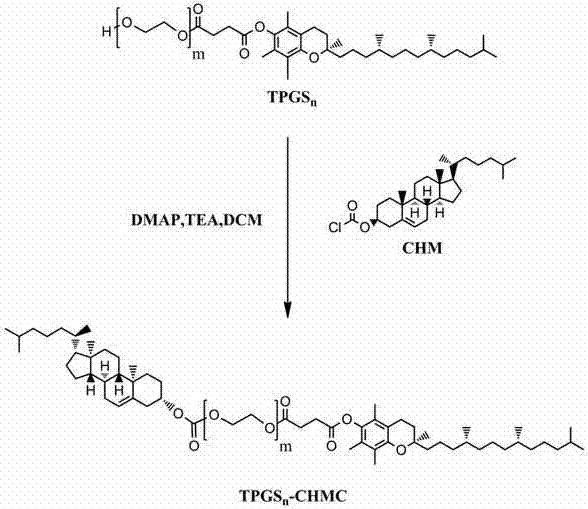
Polyethylene glycol vitamin E succinate-cholesterol carbonate and its preparation method and application
ActiveCN104844790BLow toxicityLow critical micelle concentrationOrganic active ingredientsPharmaceutical non-active ingredientsCholesterolPolyethylene glycol
The present invention relates to polymer TPGSn-cholesterol carbonate and its preparation method and application. The polymer takes TPGSn as the basic skeleton and is obtained by linking cholesterol at the hydroxyl end through a carbonate bond. The preparation method is as follows: take TPGSn and place it in a closed container, add a basic catalyst and an acid-binding agent under nitrogen, and slowly add a dichloromethane solution containing cholesteryl chloromethyl ester dropwise. Stir and mix in an ice-water bath for 5-30 min, then place it at room temperature for reaction, and remove the solvent under reduced pressure after the reaction to obtain a crude product; add an appropriate amount of distilled water to the obtained crude product, extract three times with dichloromethane, and then successively use 100 mM hydrochloric acid , saturated sodium chloride and ice water for 3 times, and precipitated by ice n-hexane to obtain a white wax; the resulting white wax can be refined by repeated precipitation with n-hexane. The polymer has good biocompatibility and biodegradability, and also has the advantages of low critical micelle concentration, good dilution stability, strong P-glycoprotein inhibitory effect, simple synthesis process, wide application range, and low cost. .
Owner:SHENYANG PHARMA UNIVERSITY +1
Preparation method and application of nanometer particles of taxane drugs
ActiveCN103263672BImprove stabilityLow costOrganic active ingredientsPowder deliveryDiffusion methodsOrganic solvent
The invention discloses a preparation method of a nanometer preparation of taxane drugs. The nanometer particles prepared by utilizing an emulsion diffusion method comprise the taxane drugs, copolymer carrier materials and emulgators. The preparation method comprises the steps of: dissolving the taxane drugs and the copolymer carrier materials into an organic phase mutually soluble with water, then dropwise adding the solution into a mixed solution of water in which the emulgators are dissolved and ethanol, stirring and mixing to form emulsion, and forming nanometer particles after removing organic solvents and solidifying. According to the nanometer particles prepared by utilizing the preparation method, the particle sizes are very uniform, the average particle size is 80-120 nm, the encapsulation efficiency is high, the stability is good, the preparation process is simple and controllable, and the scaled-up production is liable. The nanometer particles prepared by utilizing the preparation method can be applied to the treatment of malignant tumors and have obvious tumor inhibition effects.
Owner:HANGZHOU PUSH KANG BIOTECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com