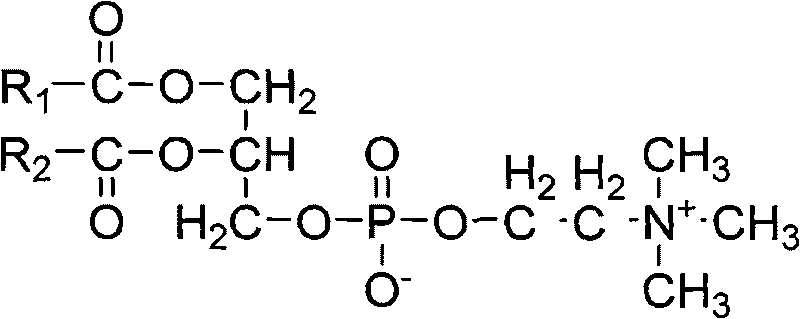
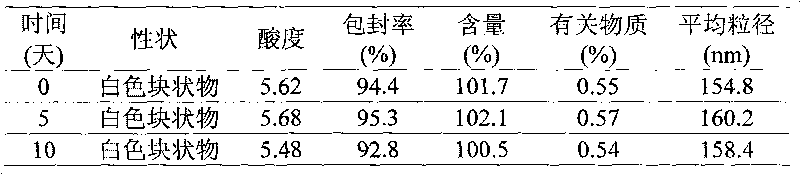
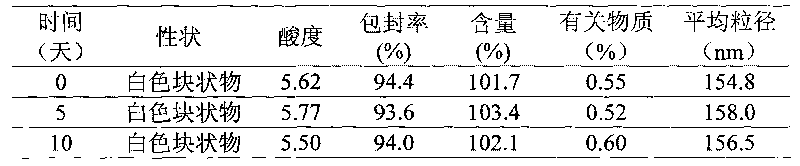
Docetaxel nano-particle composition
A docetaxel and nanoparticle technology, applied in the directions of drug combination, drug delivery, organic active ingredients, etc., can solve the problems of health hazards, high price, and low negative phospholipid content in patients with cardiovascular and cerebrovascular diseases, and improve drug safety. , low price, high encapsulation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Prescription: Docetaxel 1g
[0037]Hydrogenated Soy Lecithin 70g
[0038] 0.01NpH6.5 phosphate buffer (sodium dihydrogen phosphate or??, should be specific) 870ml
[0039] Preparation Process:
[0040] 1. Dissolve the prescription amount of docetaxel and hydrogenated soybean lecithin in 80ml of ethanol as the organic phase;
[0041] 2. Take 870ml of 0.01N pH6.5 phosphate buffer, heat it to 65°C and use it as the water phase;
[0042] 3. Slowly inject the organic phase into the water phase under nitrogen protection and stirring, and stir thoroughly for 30 minutes to obtain the nanoparticle suspension;
[0043] 4. Pass the nanoparticle suspension into a high-pressure homogenizer to homogenize for 5 times, then sterilize and filter, and pack aseptically.
Embodiment 2
[0045] Prescription: Docetaxel 1g
[0047] Sucrose 60g
[0048] Preparation Process:
[0049] 1. Dissolve the prescription amount of docetaxel and egg yolk lecithin in 22ml of ethanol as the organic phase;
[0050] 2. Take the prescribed amount of sucrose, add 230ml of water for injection to dissolve it as the water phase;
[0051] 3. Heat the water phase to 40°C at a constant temperature, slowly inject the organic phase into it under nitrogen protection and stirring, and shear for 30 minutes to obtain the nanoparticle suspension;
[0052] 4. Pass the nanoparticle suspension into a high-pressure homogenizer for homogenization 5 times, sterilize and filter, divide into vials for freeze-drying, and pack.
Embodiment 3
[0054] Prescription: Docetaxel 1g
[0055] Hydrogenated Soy Lecithin 25g
[0056] Soy Lecithin 15g
[0057] Maltose 45g
[0058] Preparation Process:
[0059] 1. Dissolve the prescription amount of docetaxel, hydrogenated soybean lecithin and soybean lecithin in 35ml ethanol as the organic phase;
[0060] 2. Take the prescribed amount of maltose, add 450ml of water for injection to dissolve it as the water phase;
[0061] 3. Heat the water phase to 70°C at a constant temperature, slowly inject the organic phase into it under nitrogen protection and stirring, and then shear for 20 minutes to obtain the nanoparticle suspension;
[0062] 4. Pass the nanoparticle suspension into the extruder and extrude it for 6 times, then sterilize and filter, put it into vials for freeze-drying, and pack.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com