Amphiphilic copolymer-maytansinoids covalently coupled drug, preparation method and application
An amphiphilic copolymer and maytansine technology, applied in the field of medicine and chemical industry, can solve the problems of high cost of synthetic process products, low drug loading, hidden dangers of drug safety, etc., and achieve good market prospects and clinical application value. , low preparation cost, good safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
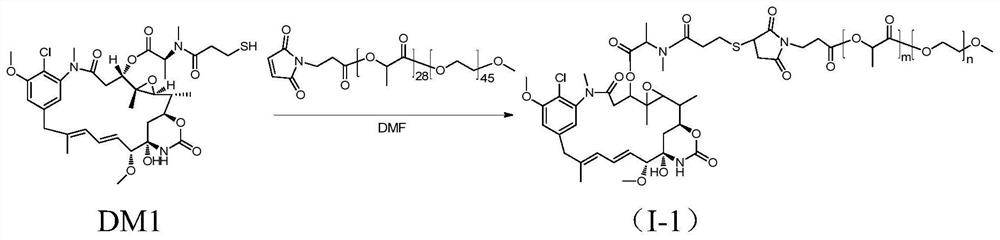
[0069] The synthesis of methoxypolyethylene glycol-polylactic acid-maleimide-maytansinoid coupling drug I-1, the synthetic route is as follows figure 1 shown.
[0070] Add mPEG(2K)-PLA(2K)-MAL (purchased from Advanced Polymer Materials, Canada, m=28, n=45, 110.7mg, 0.027mmol) and DM1 (20mg, 0.027mmol) into a 100mL round bottom flask, and use 3 mL of anhydrous DMF (dimethylformamide) was dissolved. At 37°C, the reaction was carried out under magnetic stirring for 20 hours. After the reaction was completed, DMF was removed by rotary evaporation, and the solid was dissolved in dichloromethane, and purified by column chromatography (DCM:MeOH=20:1) to obtain product I-1 (80.2 mg, yield 61%).
[0071] Product I-1 1 The H NMR nuclear magnetic data is as follows:
[0072] 1 H NMR (400MHz, CDCl 3 ): δ0.80(s,3H),1.24(s,1H),1.26(s,3H),1.52(s,3H),1.55-1.57(d,84H,J=6.8),1.64(s,3H ),2.85(s,3H),3.01-3.02(d,1H,J=4.80),3.06-3.14(t,2H),3.20-3.21(d,3H,J=2.0),3.36(s,3H) ,3.38(s,3H),3.46-3...
Embodiment 2
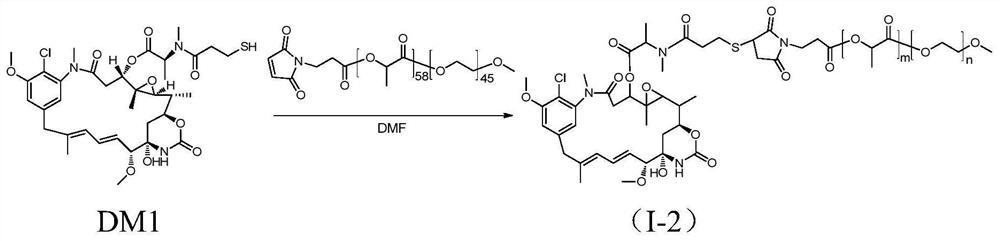
[0074] The synthesis of methoxypolyethylene glycol-polylactic acid-maleimide-maytansinoid coupling drug I-2, the synthetic route is as follows figure 2 shown.
[0075] Add mPEG(2K)-PLA(4.2K)-MAL (purchased from Advanced Polymer Materials, Canada, m=58, n=45, 300mg, 0.048mmol) and DM1 (35.1mg, 0.048mmol) into a 100mL round bottom flask, Dissolve with 3 mL of anhydrous DMF (dimethylformamide). At 37°C, the reaction was carried out under magnetic stirring for 20 hours. After the reaction was completed, DMF was removed by rotary evaporation, and the solid was dissolved in dichloromethane, and purified by column chromatography (DCM:MeOH=40:1) to obtain product I-2 (227.8 mg, yield 68%).
[0076] Product I-2 1 The H NMR nuclear magnetic data is as follows:
[0077] 1 H NMR (400MHz, CDCl 3 ): δ0.80(s,3H),1.23(s,1H),1.26(s,4H),1.52(s,3H),1.55-1.59(m,174H),1.64(s,3H),2.85( s,3H),3.02-3.04(d,1H,J=9.60),3.07-3.12(t,2H),3.19-3.21(t,3H),3.36(s,3H),3.38(s,3H), 3.46-3.49 (m,2H),3.51...
Embodiment 3
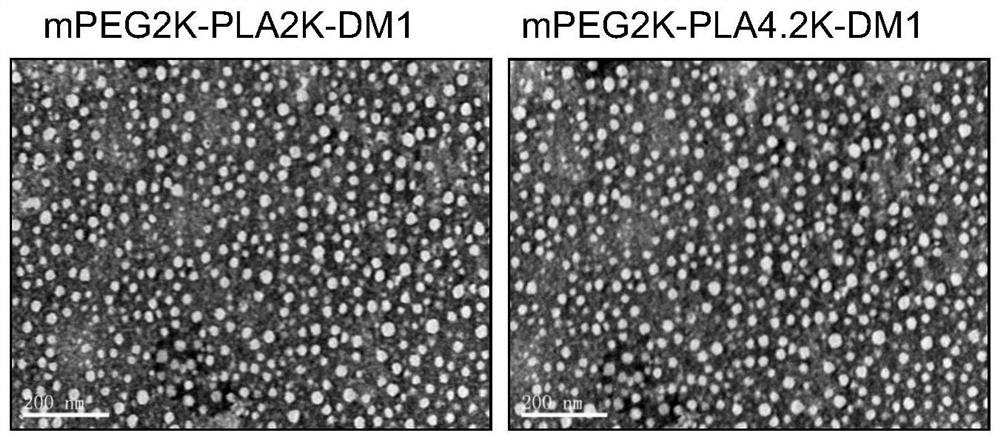
[0079] Dissolve 5 mg each of the conjugates I-1 and conjugates I-2 prepared in Example 1 and Example 2 in 2 mL of acetone, and slowly inject them into 20 mL of water to obtain nano drug. The acetone solution was removed by rotary evaporation under reduced pressure to obtain nanomedicine.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com