Polyethylene glycol vitamin E succinate-cholesterol carbonate and its preparation method and application
A technology of cholesterol carbonate and succinate, which is applied in the direction of pharmaceutical formulations, medical preparations containing non-active ingredients, medical preparations containing active ingredients, etc., to achieve high yield, targeted targeting, and large drug loading capacity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: TPGS 1000 -Synthesis of CHMC
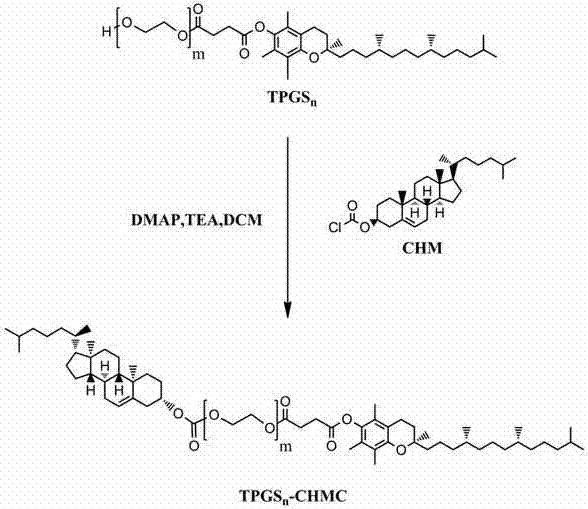
[0044] Take 1g TPGS 1000 (0.66mmol) was placed in a closed container, 41.5mg 4-lutidine (DMAP) and 142.9μL triethylamine (TEA) were added under nitrogen, and dichloride of cholesteryl chloromethyl ester (CHM, 1mmol) was slowly added dropwise 20 mL of methane (DCM) solution was stirred and mixed in an ice-water bath for 10 min, and then refluxed at 45° C. for 24 h. After the reaction was completed, the solvent was removed under reduced pressure. (Synthetic process such as figure 1 As shown), add a certain amount of distilled water to the obtained crude product, extract three times with dichloromethane, then wash three times with 100mM hydrochloric acid, saturated sodium chloride and ice water respectively, and obtain a milky white wax by ice n-hexane precipitation, continue to use n-hexane Repeated precipitation and purification of alkanes for 3 times to obtain TPGS 1000 - Pure CHMC polymer.
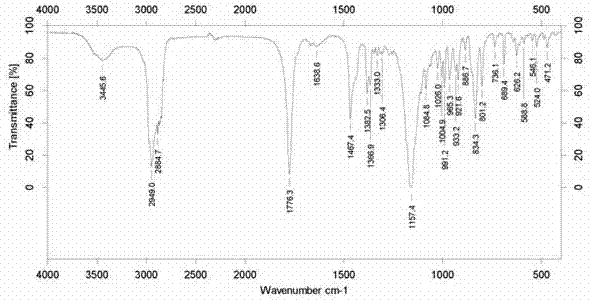
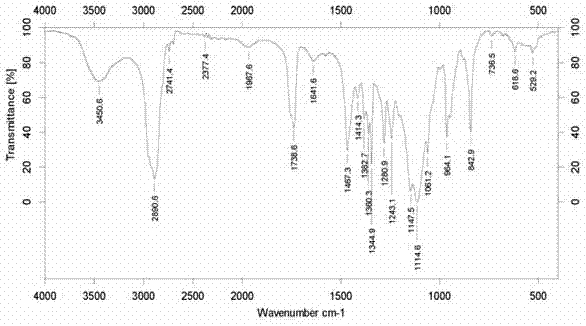
[0045] During the reaction, sil...
Embodiment 2
[0051] Example 2: TPGS 400 -Synthesis of CHMC
[0052] Take 0.6g TPGS 400 (0.66mmol) was placed in a closed container, 41.5mg 4-lutidine (DMAP) and 142.9μL triethylamine (TEA) were added under nitrogen, and dichloride of cholesteryl chloromethyl ester (CHM, 1mmol) was slowly added dropwise 20 mL of methane (DCM) solution was stirred and mixed in an ice-water bath for 10 min, and then refluxed at 45° C. for 24 h. After the reaction was completed, the solvent was removed under reduced pressure. (Synthetic process such as figure 1 As shown), add a certain amount of distilled water to the obtained crude product, extract three times with dichloromethane, then wash three times with 100mM hydrochloric acid, saturated sodium chloride and ice water respectively, and obtain a milky white wax by ice n-hexane precipitation, continue to use n-hexane Repeated precipitation and purification of alkanes for 3 times to obtain TPGS 400 - Pure CHMC polymer.
[0053] During the reaction, sili...
Embodiment 3
[0059] Example 3: TPGS 6000 -Synthesis of CHMC
[0060] Take 4.3g TPGS 6000 (0.66mmol) was placed in a closed container, 41.5mg 4-lutidine (DMAP) and 142.9μL triethylamine (TEA) were added under nitrogen, and dichloride of cholesteryl chloromethyl ester (CHM, 1mmol) was slowly added dropwise 20 mL of methane (DCM) solution was stirred and mixed in an ice-water bath for 10 min, and then refluxed at 45° C. for 24 h. After the reaction was completed, the solvent was removed under reduced pressure. (Synthetic process such as figure 1 As shown), add a certain amount of distilled water to the obtained crude product, extract three times with dichloromethane, then wash three times with 100mM hydrochloric acid, saturated sodium chloride and ice water respectively, and obtain a milky white wax by ice n-hexane precipitation, continue to use n-hexane Repeated precipitation and purification of alkanes for 3 times to obtain TPGS 6000 - Pure CHMC polymer.
[0061] During the reaction, s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com