Meclofenoxate hydrochloride preparation freeze-drying technique and preparation method thereof
A technology for meclofen axetil hydrochloride and freeze-dried preparations is applied in the field of medicinal preparations and preparations thereof, which can solve the problems of easy hydrolysis of meclofen axetil hydrochloride, inability to solve the problems of moisture absorption in storage, reduced clinical efficacy and the like, and achieves a simple and easy process. The production method is reasonable and reliable, and has no toxic and side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
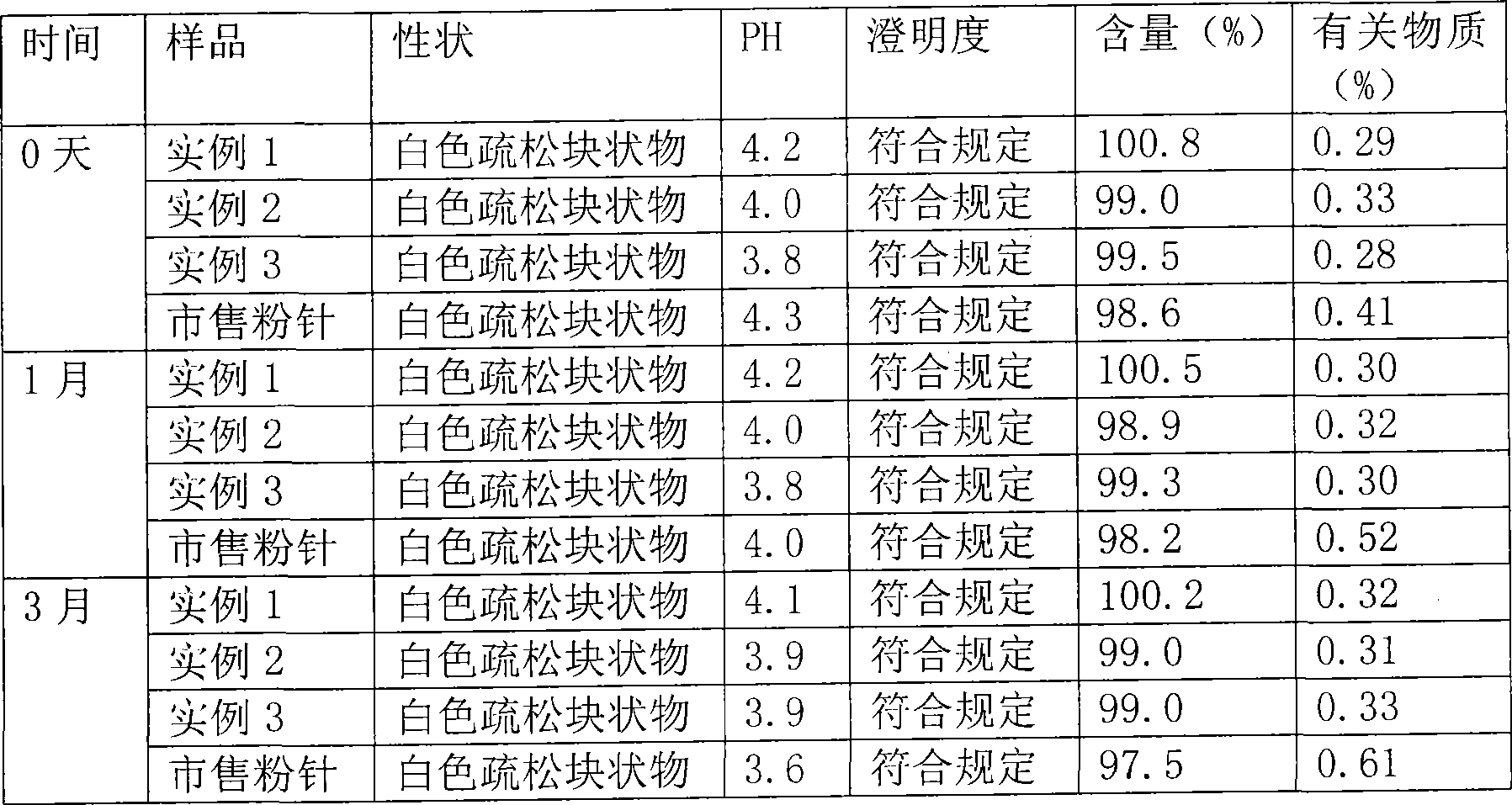
[0021] The freeze-dried preparation of meclofenoxate hydrochloride for injection and the preparation process thereof comprise the freeze-dried preparation made of meclofenoxate hydrochloride and mannitol. The parts by weight are 10 parts by weight of meclofenoxate hydrochloride, 17 parts by mannitol, pH is 4.1, and the properties are white or off-white freeze-dried loose block or powder. The preparation process is as follows: take the prescribed amount of mannitol and add water for injection, stir to dissolve; add meclofenoxate hydrochloride to dissolve, add water for injection to constant volume, absorb with 0.01% activated carbon, and filter; use 0.45 μm microporous membrane for primary filtration Then use a 0.22 μm microporous membrane for fine filtration; measure the solution content, and fill each vial with about 2.0ml according to the preparation specifications; freeze-dry, stopper, cap, and pack after passing the quality inspection. The packaging specification is 0.1g. ...
Embodiment 2
[0023] The freeze-dried preparation of meclofenoxate hydrochloride for injection and the preparation process thereof comprise the freeze-dried preparation made of meclofenoxate hydrochloride and mannitol. The parts by weight are 20 parts by weight of meclofenoxate hydrochloride, 10 parts by mannitol, pH is 3.9, and the properties are white or off-white freeze-dried loose block or powder. The preparation process is as follows: take the prescribed amount of mannitol and add water for injection, stir to dissolve; add meclofenoxate hydrochloride to dissolve, add water for injection to constant volume, absorb with 0.01% activated carbon, and filter; use 0.45 μm microporous membrane for primary filtration Then use a 0.22 μm microporous membrane for fine filtration; measure the solution content, and fill each vial with about 2.0ml according to the preparation specifications; freeze-dry, stopper, cap, and pack after passing the quality inspection. The packaging specification is 0.2g. ...
Embodiment 3
[0025] The freeze-dried preparation of meclofenoxate hydrochloride for injection and the preparation process thereof comprise the freeze-dried preparation made of meclofenoxate hydrochloride and mannitol. The parts by weight are 25 parts by weight of meclofenoxate hydrochloride, 10 parts by mannitol, the pH is 4.0, and the properties are white or off-white freeze-dried loose block or powder. The preparation process is as follows: take the prescribed amount of mannitol and add water for injection, stir to dissolve; add meclofenoxate hydrochloride to dissolve, add water for injection to constant volume, absorb with 0.01% activated carbon, and filter; use 0.45 μm microporous membrane for primary filtration Then use a 0.22 μm microporous membrane for fine filtration; measure the solution content, and fill each vial with about 2.0ml according to the preparation specifications; freeze-dry, stopper, cap, and pack after passing the quality inspection. The packaging specification is 0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com