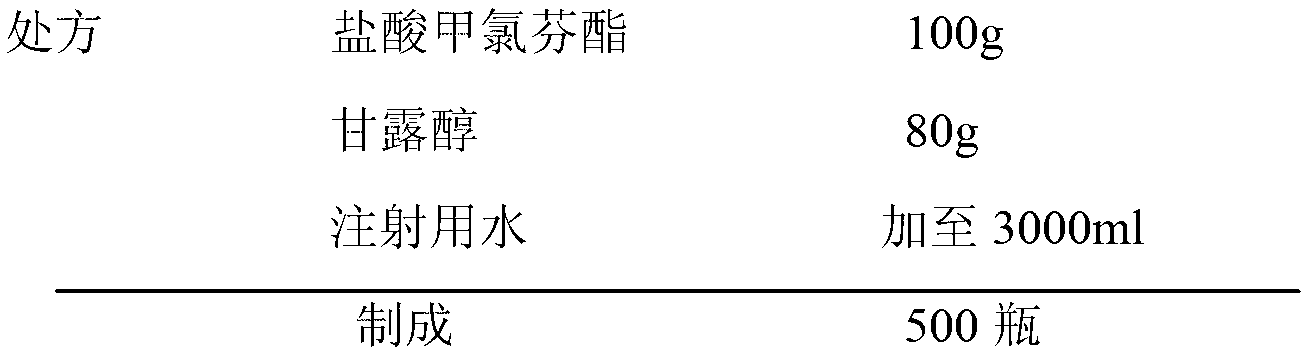
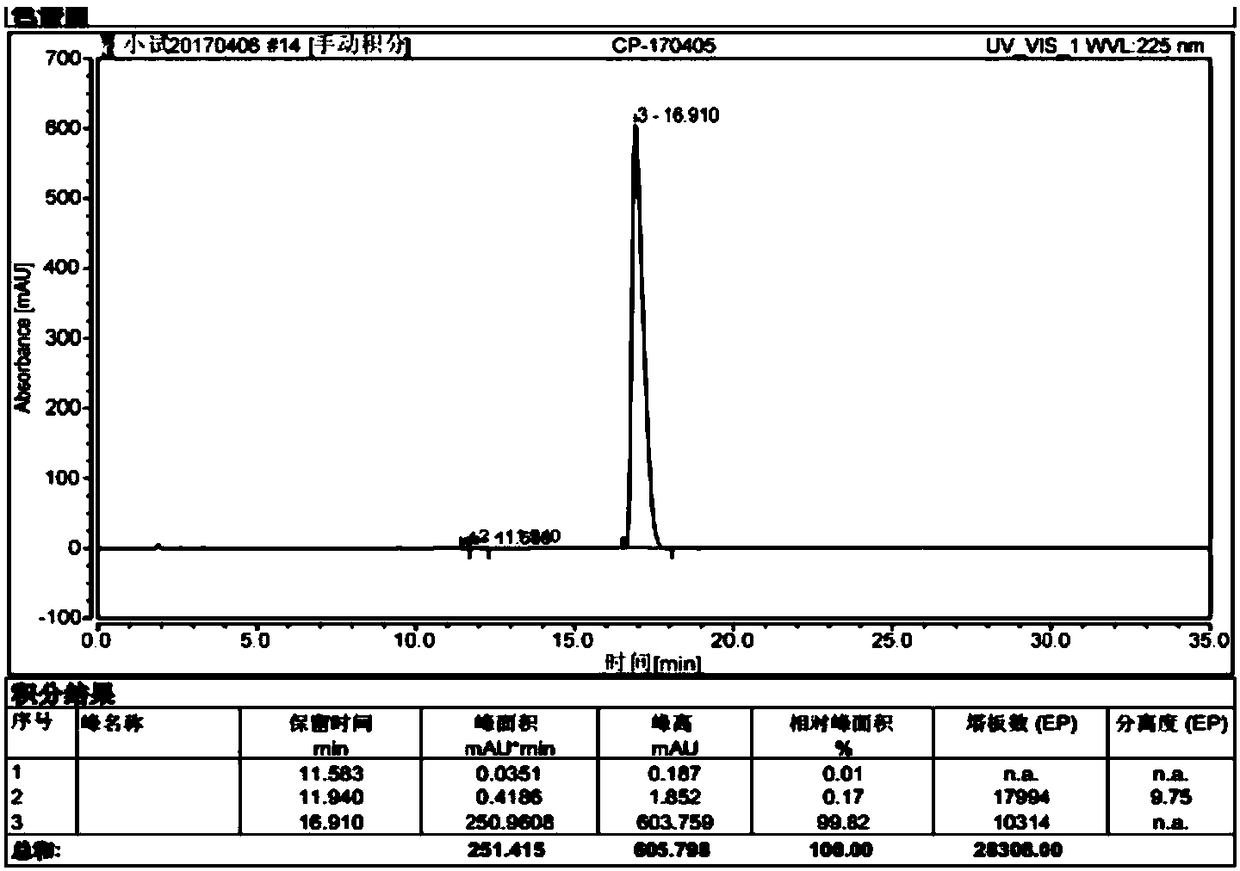
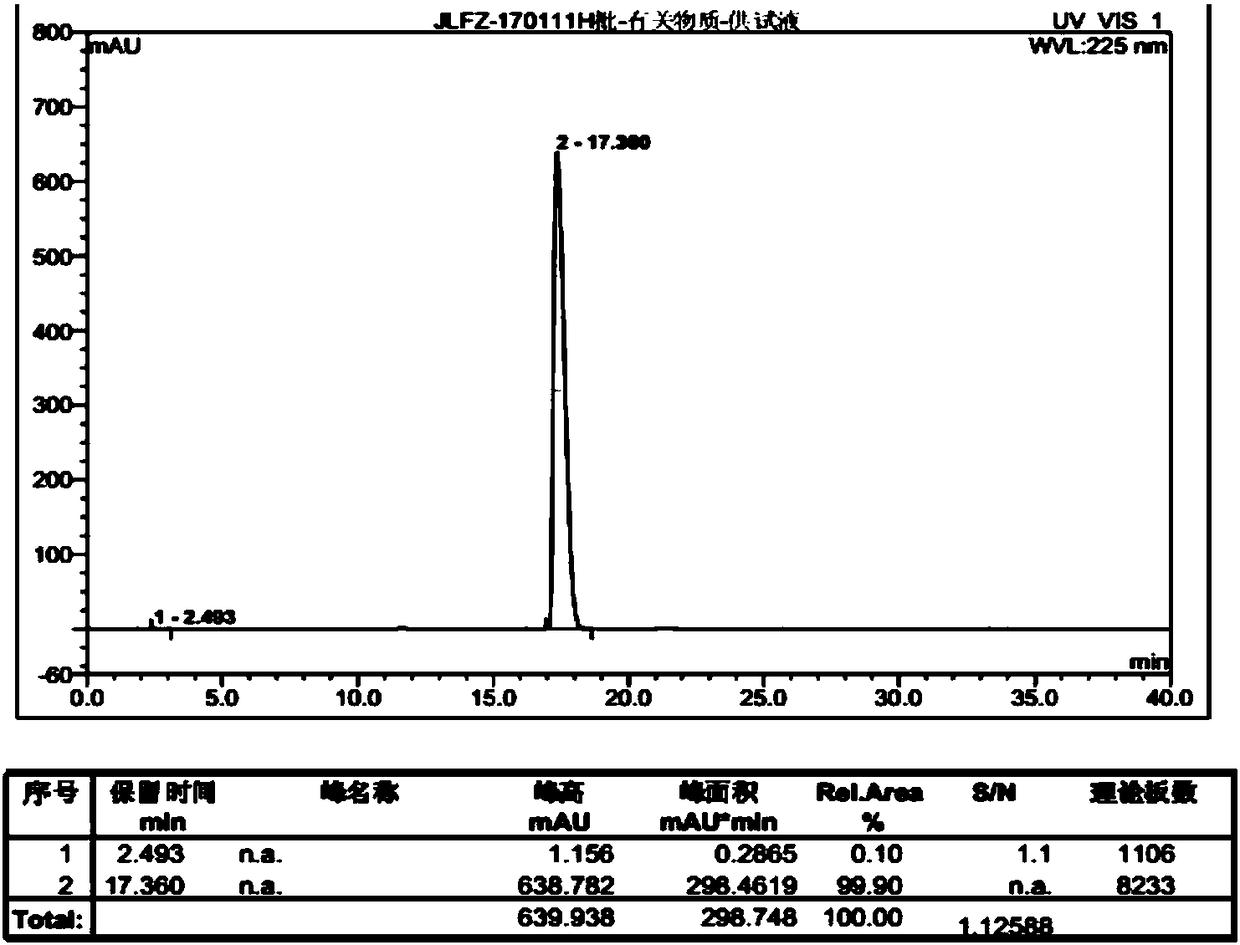
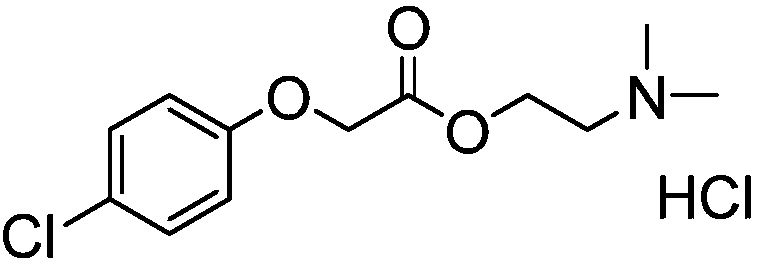
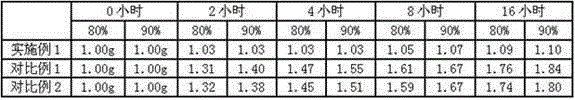
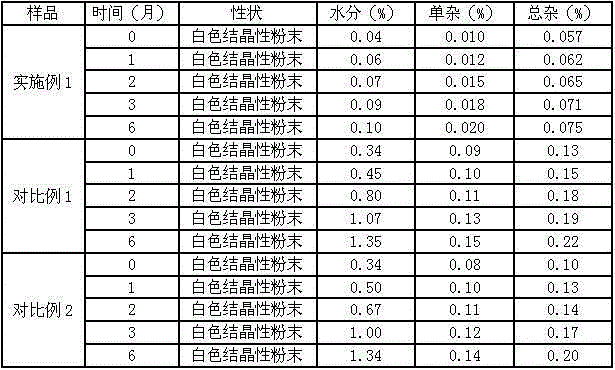
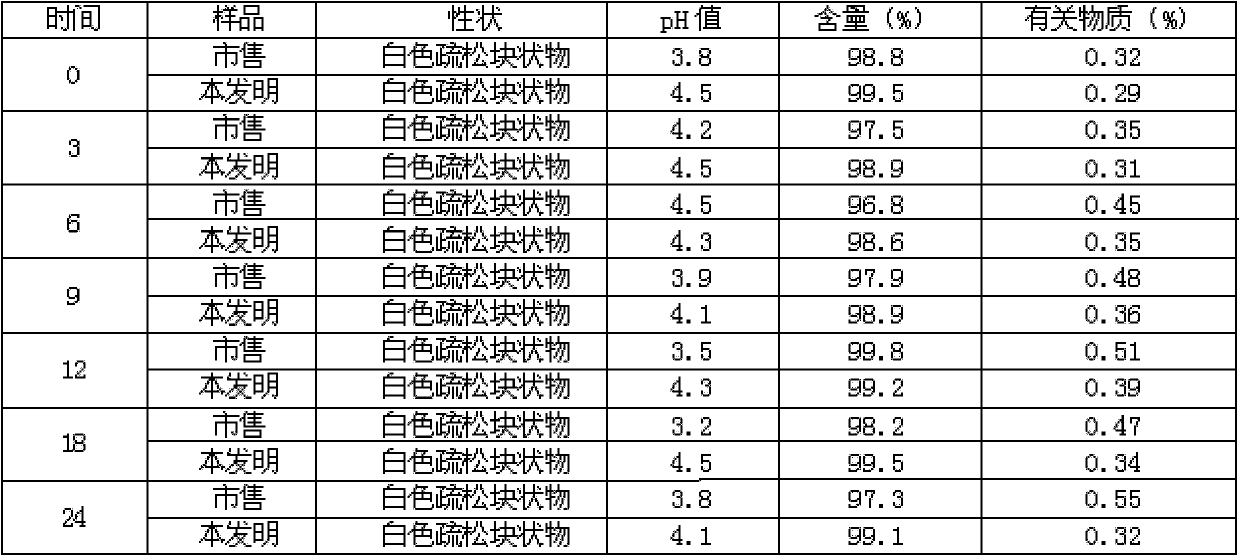
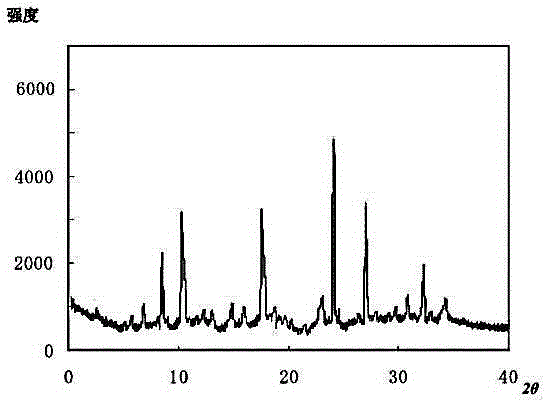
Patents
Literature
32 results about "Meclofenoxate Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
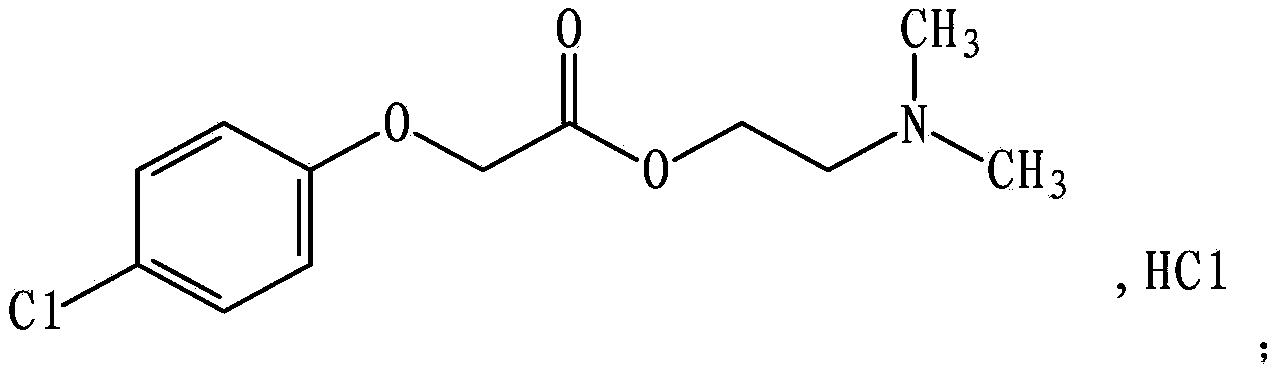
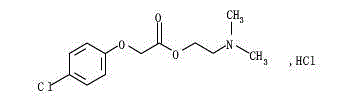
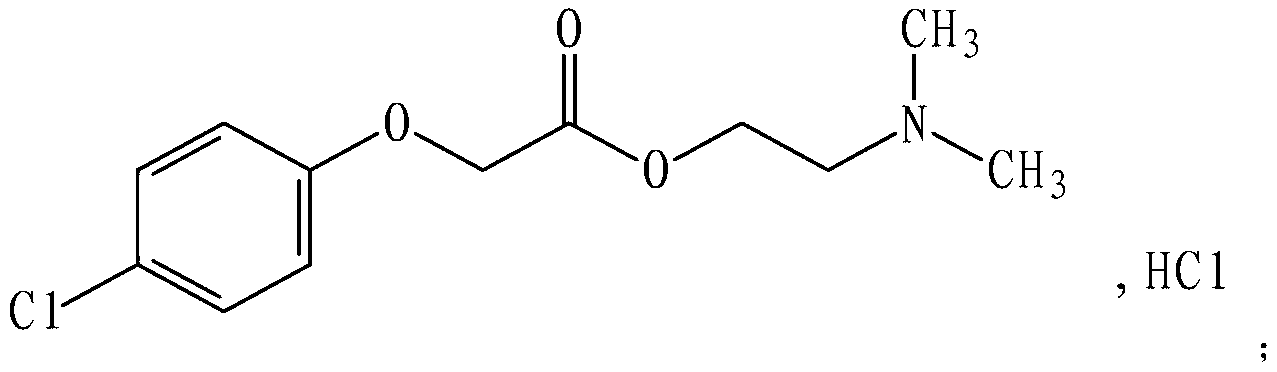
Meclofenoxate hydrochloride compound and pharmaceutical composition thereof
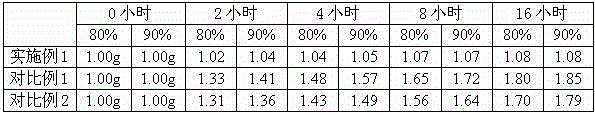
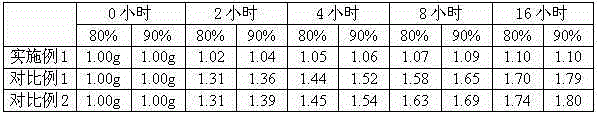
ActiveCN103214382BLow impurity contentGood storage stabilityOrganic active ingredientsNervous disorderFreeze-dryingStructural formula
Owner:SHANXI C&Y PHARMACEUTICAL GROUP CO LTD
Meclofenoxate hydrochloride compound and pharmaceutical composition thereof
ActiveCN103396328BImprove stabilityLow impurity contentOrganic active ingredientsNervous disorderBULK ACTIVE INGREDIENTPharmaceutical Substances
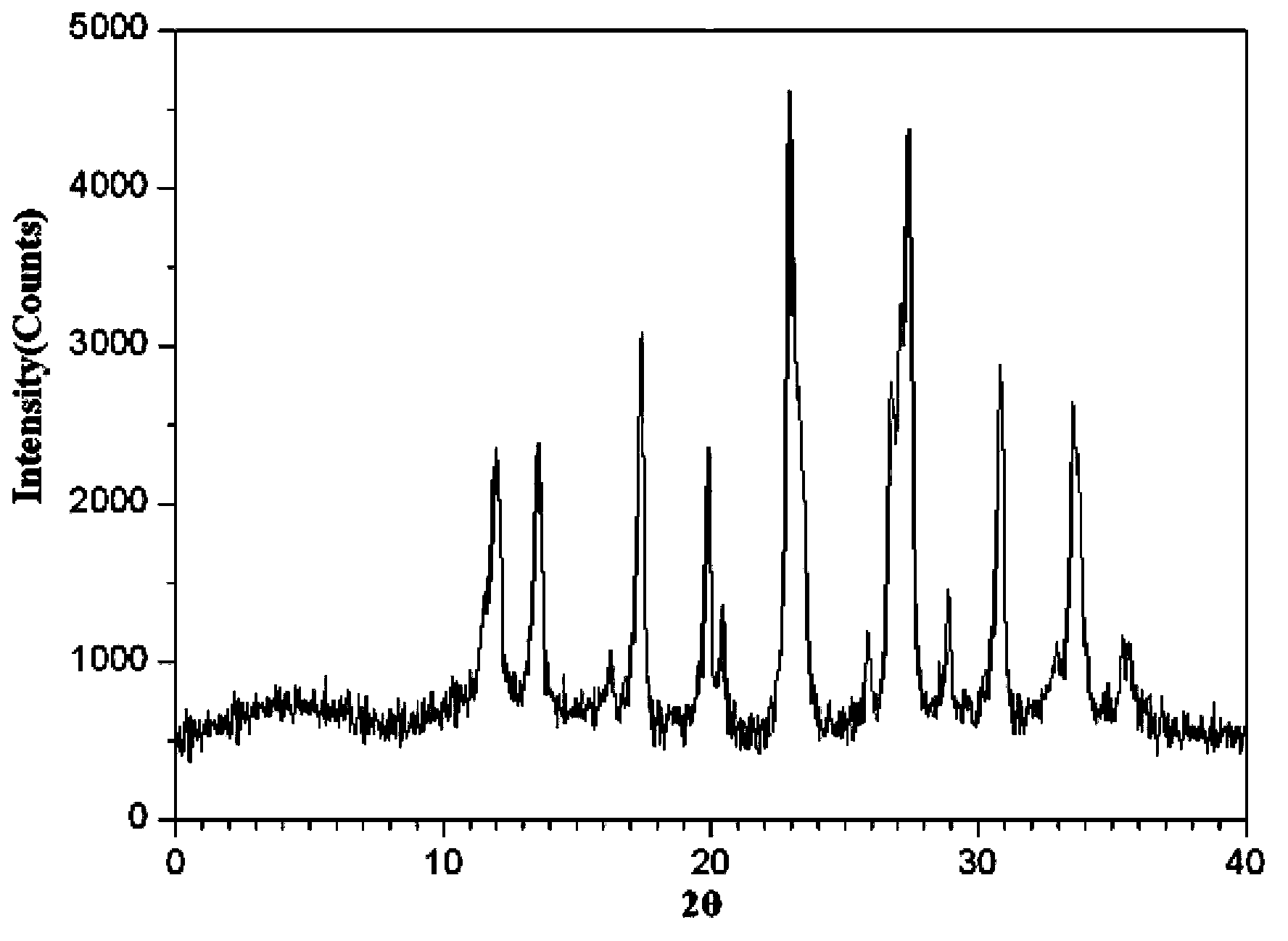
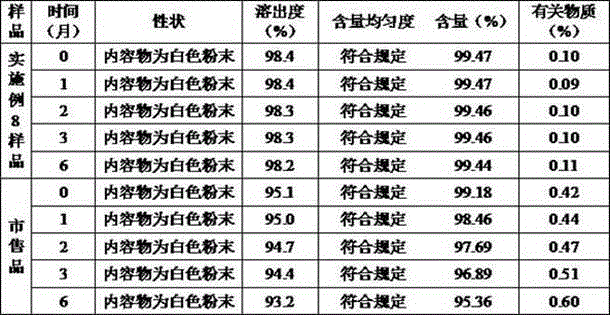
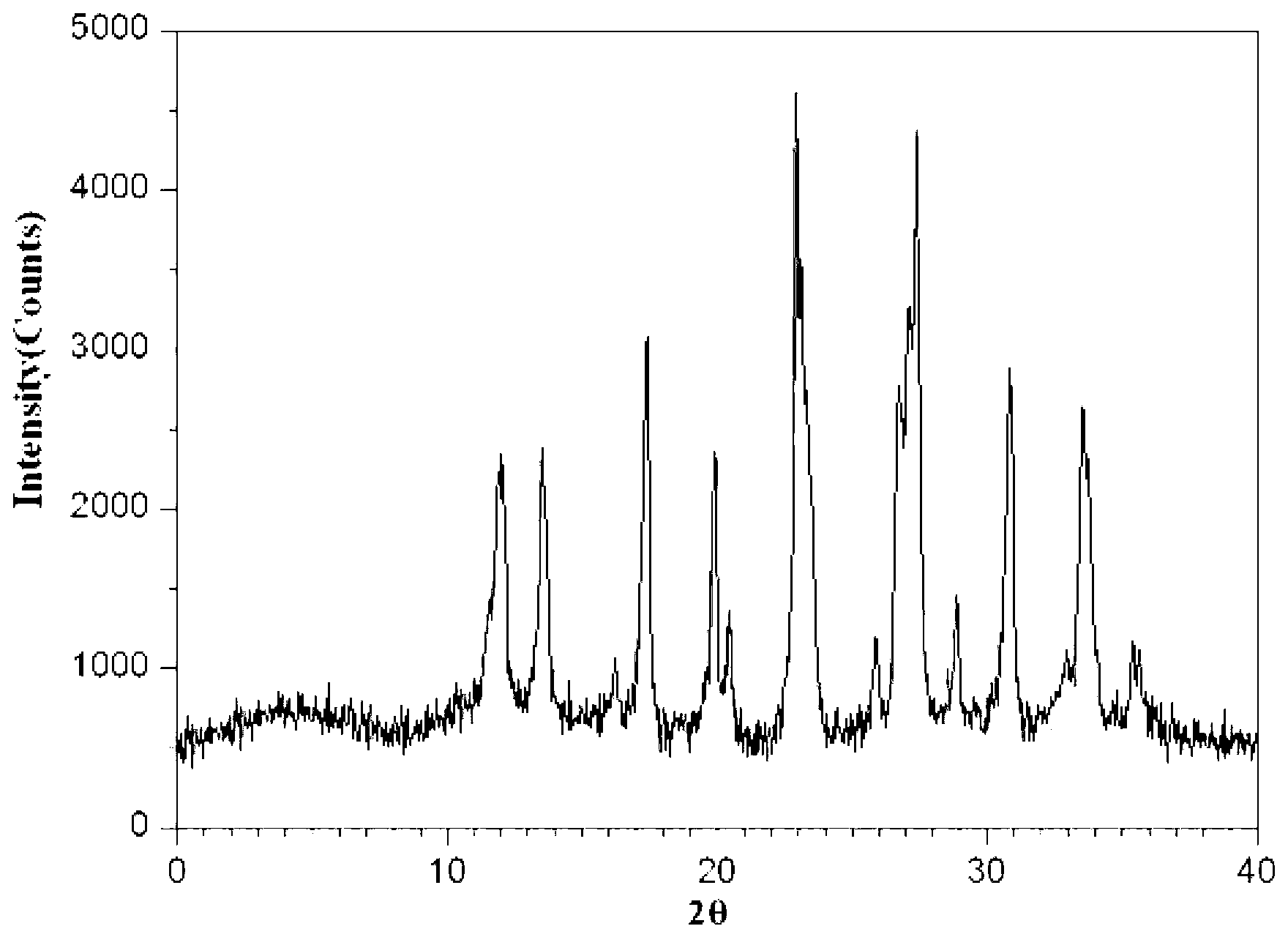
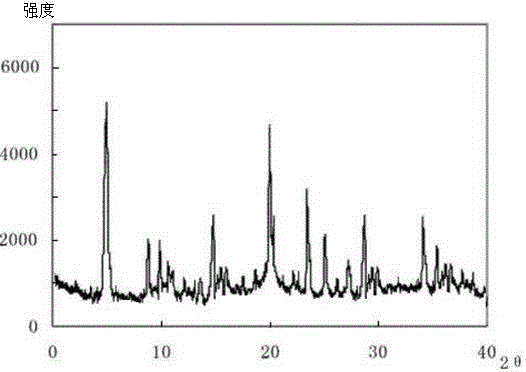
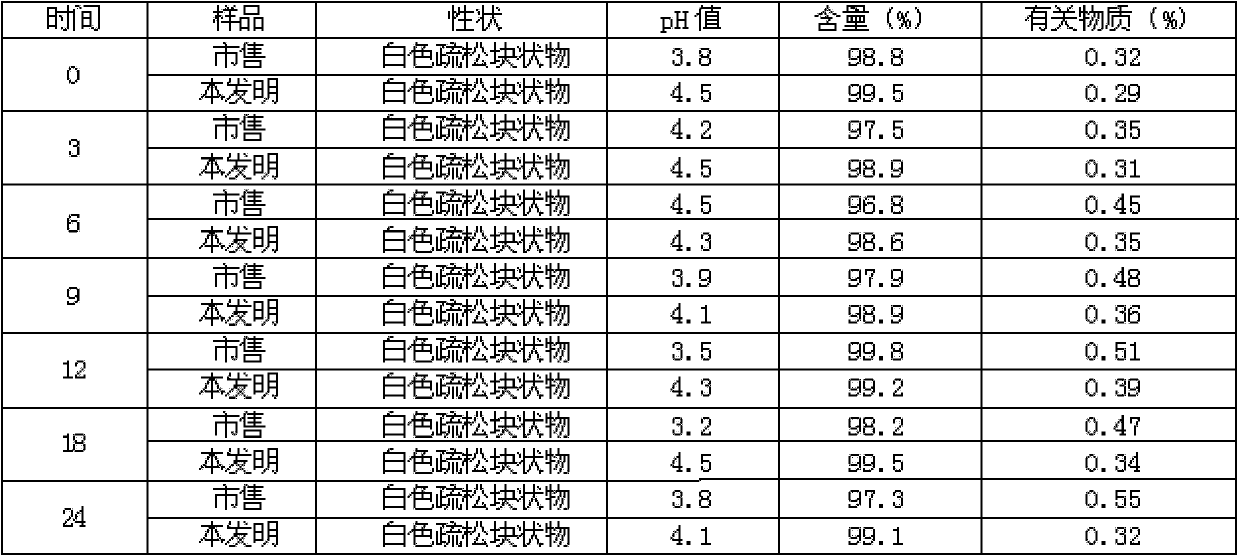
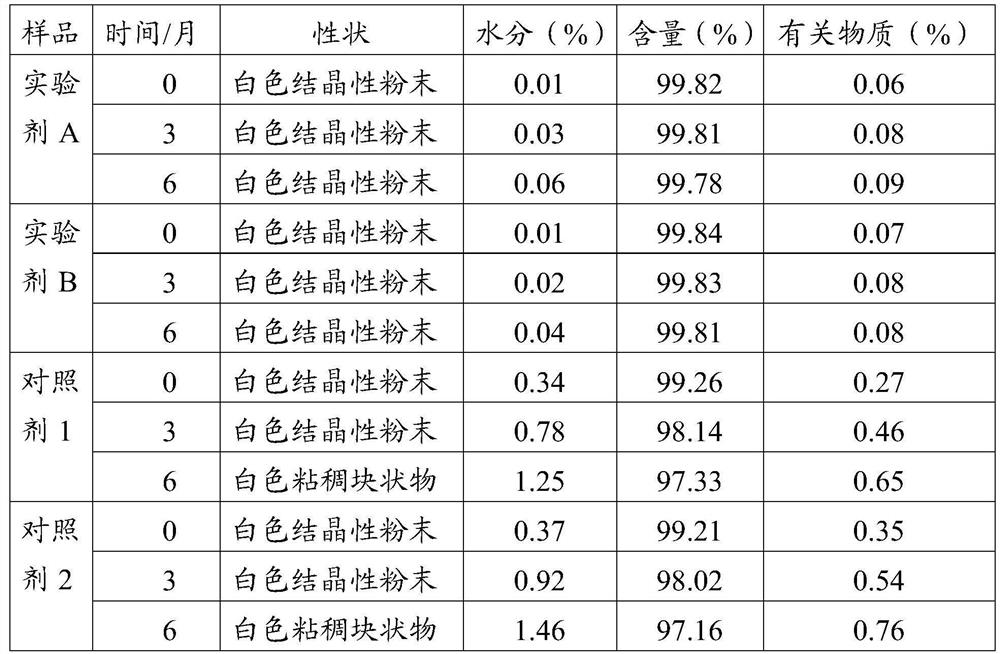
Meclofenoxate hydrochloride compound, described meclofenoxate hydrochloride compound is crystal, adopts X-ray powder diffraction to measure, and characteristic peak is 6.1°, 7.7°, 9.3°, 12.2°, 14.3°, 17.8°, 18.9°, 20.5°, 22.2°, 23.3°, 24.5°, 25.8°, 27.0° display. The pharmaceutical composition preparation of the above-mentioned meclofenoxate hydrochloride compound, the described pharmaceutical composition preparation is a dispersible tablet, a capsule, a freeze-dried powder injection and a sterile powder for injection. In addition to stable quality, the meclofenoxate hydrochloride compound of the present invention also has the characteristics of excellent fluidity. The active ingredient prepared by the invention is the dispersible tablet, capsule and freeze-dried powder of meclofenoxate hydrochloride prepared by the invention, which has good stability and low impurity content.
Owner:HUNAN WUZHOUTONG PHARMA
Injection containing meclofenoxate hydrochloride and its preparing method
InactiveCN100998585ASolve the problem of hydrolysisImprove stabilityOrganic active ingredientsNervous disorderPEG 400Alcohol
An injection of meclofenoxate hydrochloride and its preparing process which features use of non-water solvent chosen from alcohol, propanediol, glycerin, polyethanediol-300 and polyethanediol-400, and use of filter sterilizing method or wet-heat sterilizing method for high stability are disclosed.
Owner:ZHENDI MEDICINE CONSULTATION NANJING
Meclofenoxate hydrochloride compound and pharmaceutical composition thereof
ActiveCN103214382AHigh lattice energyImprove stabilityOrganic active ingredientsNervous disorderStructural formulaPowder diffraction
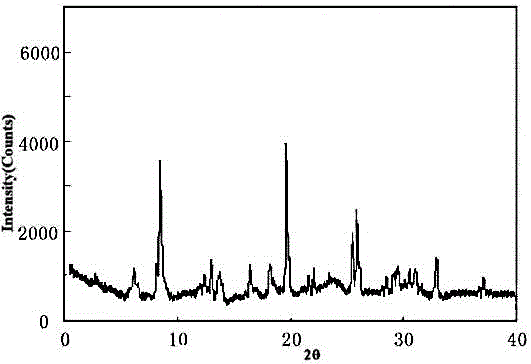
The invention provides a meclofenoxate hydrochloride compound. A structural formula of the meclofenoxate hydrochloride compound is as shown in the specification, and the meclofenoxate hydrochloride compound uses an X-ray powder diffraction spectrogram obtained through Cu-K alpha ray measurement, as shown in Figure 1 in the specification. The invention further provides a preparation method of the meclofenoxate hydrochloride compound, and a pharmaceutical composition containing the meclofenoxate hydrochloride compound. The pharmaceutical composition of the meclofenoxate hydrochloride compound is an aseptic powder injection, a freeze-drying powder injection, a dispersible tablet, a tablet or oral liquid. Compared with the prior art, the meclofenoxate hydrochloride compound and the pharmaceutical composition of the meclofenoxate hydrochloride compound have better storage stability and fluidity, and greatly improve the medication safety of a patient.
Owner:SHANXI C&Y PHARMACEUTICAL GROUP CO LTD
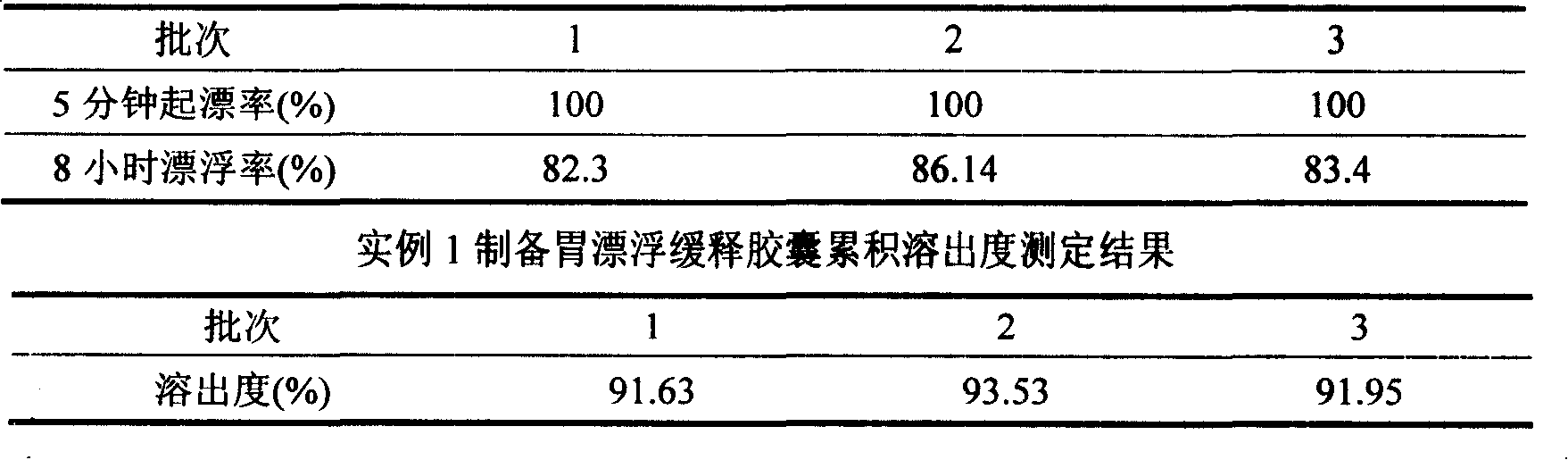
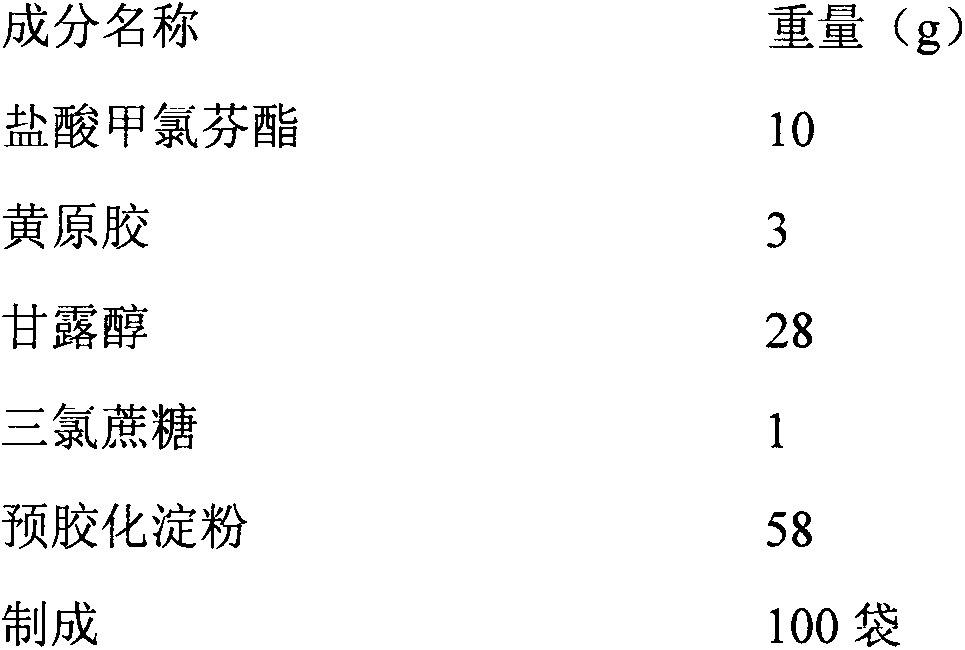
Meclofenoxate hydrochloride stomach-floating sustained release capsule and preparing method thereof
InactiveCN101229149AImprove stabilityRelease stabilityOrganic active ingredientsNervous disorderSustained Release CapsuleCurative effect
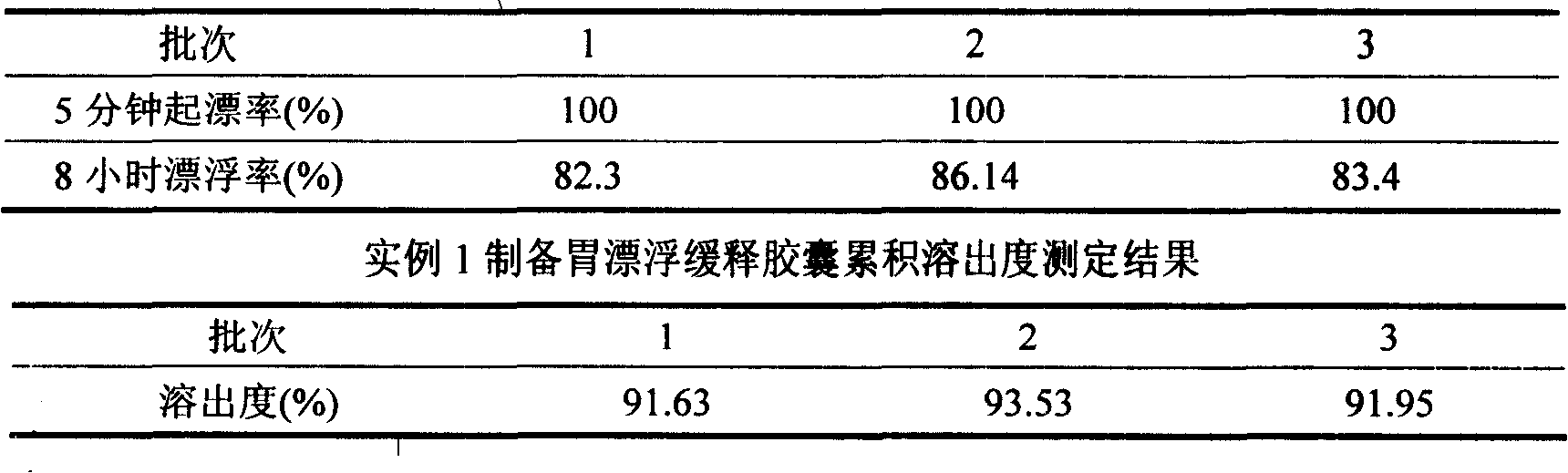
The invention discloses a meclofenoxate hydrochloride intra-gastric floating sustained release capsule. The invention adopts the intra-gastric floating sustained release technique to prevent the bad effect to the stability of the medicine in capsules caused by the elevation of the PH value in gastrointestinal tract, avoid intestinal juice in human body from retrograding the meclofenoxate hydrochloride in capsules, which ensures that the medicine can release steadily, slowly and completely. The capsule of the invention should be taken twice every day and can float continuously in stomach for more than 8 hours; the medicine can be released for 12 hours continuously and the release rate at last reaches to over 90 percent, thereby enhancing the stability of the medicine, prolonging the curing effect, greatly being convenient for patients to take medicine. The invention also provides the preparation method.
Owner:SHANGHAI PHARMA IND
Method for preparing meclofenoxate hydrochloride sterile bulk drug
InactiveCN101747215AReduce moistureQuality improvementOrganic active ingredientsNervous disorderFiltrationChemistry
The invention relates to a method for preparing a meclofenoxate hydrochloride sterile bulk drug, which is characterized in that a meclofenoxate hydrochloride sterile finished product is obtained by the following steps: dissolving meclofenoxate hydrochloride in isopropanol in a certain mixture ratio, then decoloring and carrying out aseptic filtration and decompression concentration, and then devitrifying, filtering and drying. The product prepared by the method has more stable quality and simple process, thereby being suitable for industrial big production.
Owner:YAOPHARMA CO LTD
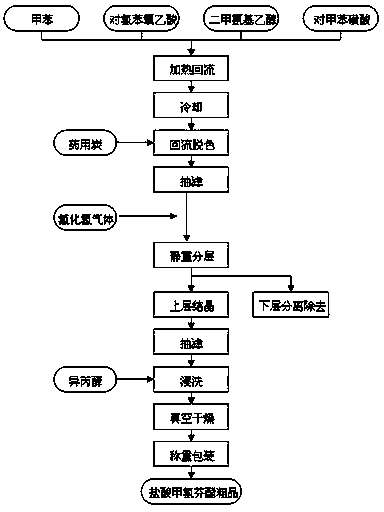
Synthetic process of crude meclofenoxate hydrochloride
ActiveCN104072382AImprove qualityReduce manufacturing costOrganic compound preparationAmino-hyroxy compound preparationRefluxPolymer science
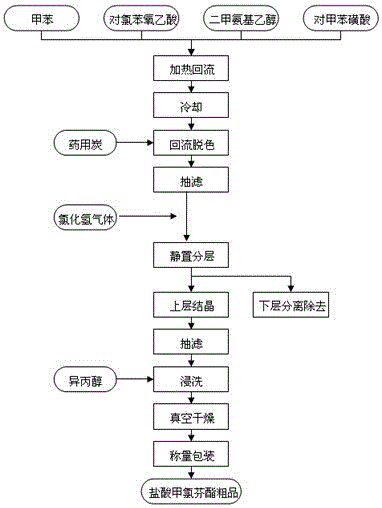
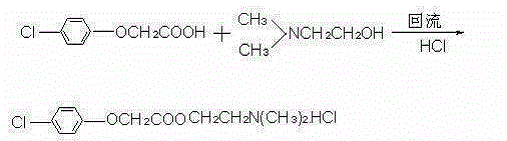
The invention discloses a synthetic process of crude meclofenoxate hydrochloride. The synthetic process comprises the following steps: checking and cleaning a detection instrument, production equipment and a working place before production, performing a heating reflux reaction on toluene, tolueneaminoethanol, p-chlorophenoxyacetic acid and toluenesulfonic acid, cooling, decolorizing, performing suction filtration on decolorizing liquid, standing and layering filter liquor, performing suction filtration, immersion cleaning and vacuum drying on upper-layer crystals to prepare crude meclofenoxate hydrochloride, and finally weighing and packaging. According to the synthetic process, high-quality crude meclofenoxate hydrochloride can be prepared, the yield is 74.0%-78.0%, a good foundation is laid for refining meclofenoxate hydrochloride, and the production cost is reduced.
Owner:JIANGSU HI STONE PHARMA
Meclofenoxate hydrochloride preparation freeze-drying technique and preparation method thereof
InactiveCN101455646AReduce hydrolysis reactionImprove bioavailabilityPowder deliveryOrganic active ingredientsMANNITOL/SORBITOLFreeze-drying
The present invention relates to a technique of lyophilized preparation of meclofenxate hydrochloride and a preparing method thereof. According to the invention, mannitol with effective dose of medicament is added with injection water and is dissolved. The meclofenxate hydrochloride with effective dose of medicament is added and mixed to uniform. The pH value is adjusted to 3-5. The obtained preparing is adsorbed with 0.01% of active carbon and is filtered. Then free drying is executed for preparing the lyophilized preparation. The meclofenxate hydrochloride in the invention is enveloped by macromolecule material and greatly reduces the hydrolytic reaction of meclofenxate hydrochloride. Therefore the lyophilized preparation of meclofenxate hydrochloride prepared by the invention has enough stability in water and can totally satisfy the requirement of clinical medicine taking. Furthermore the lyophilized preparation of meclofenxate hydrochloride prepared by the invention can be stably and slowly released. The bioavailability of meclofenxate hydrochloride is increased and transparency after re-dissolving is excellent.
Owner:朗美药业(武汉)有限公司
Pharmaceutical composition with Meclofenoxate hydrochloride capable of preventing hydrolysis
ActiveCN1951501AAvoid hydrolysisOrganic active ingredientsNervous disorderActive componentCombinatorial chemistry
The invention relates to an alcaine methyl chloroformate compound which can avoid hydrolysis. Wherein, said compound comprises active component alcaine methyl chloroformate, findings, lubricant and stabilizer, while the stabilizer can keep the compound at weak acid. And the invention uses full powder compress method to avoid hydrolysis problem.
Owner:GUANGDONG XIANQIANG PHARMA +2
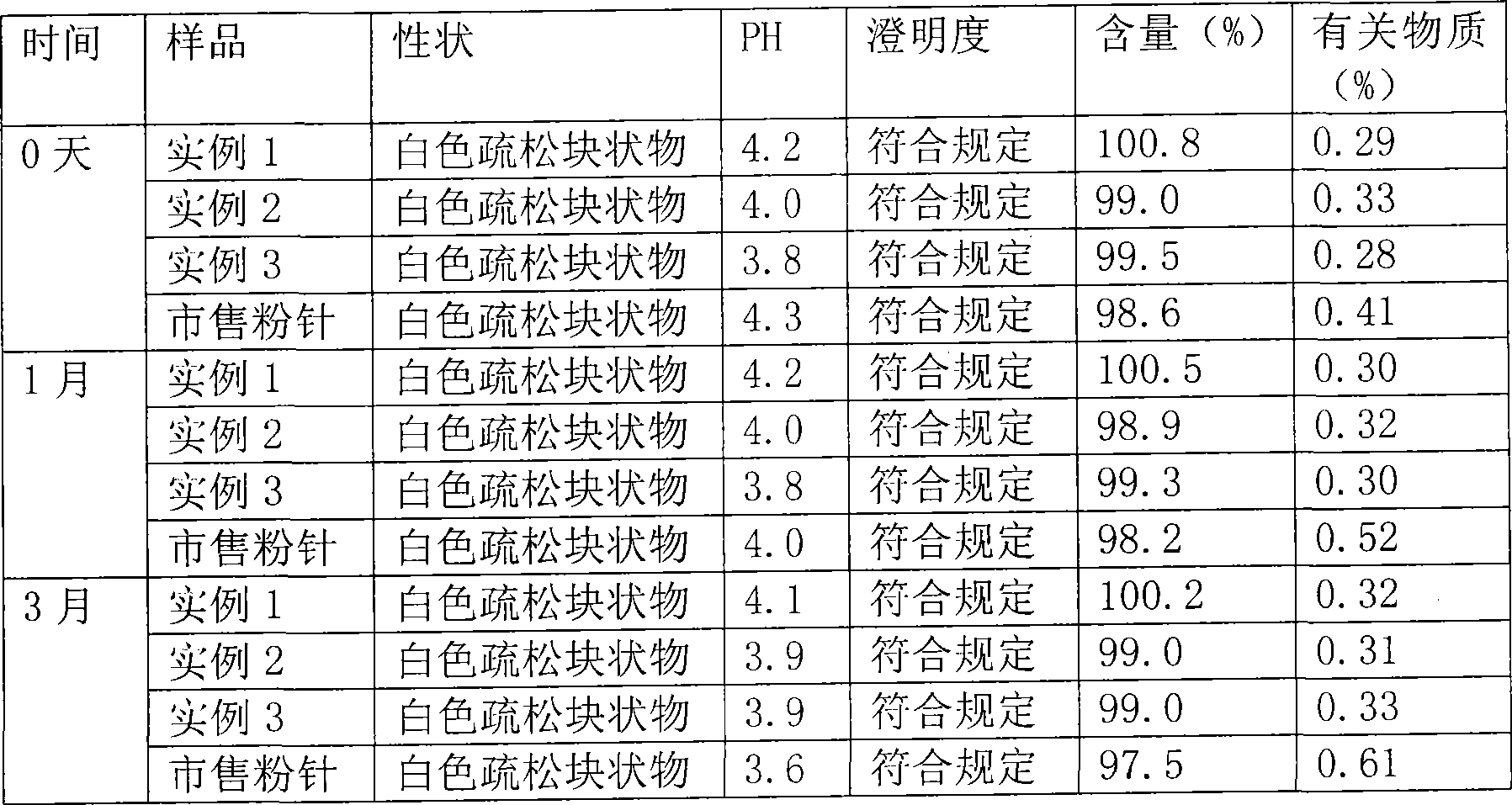
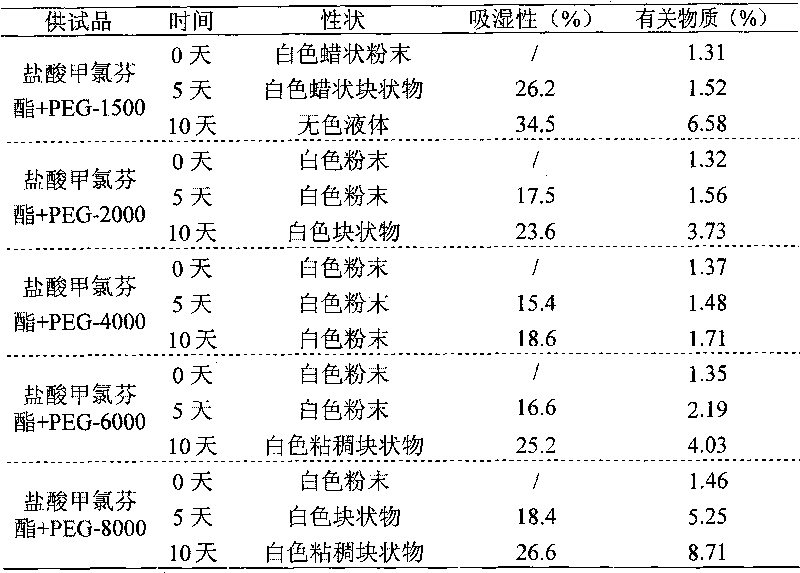
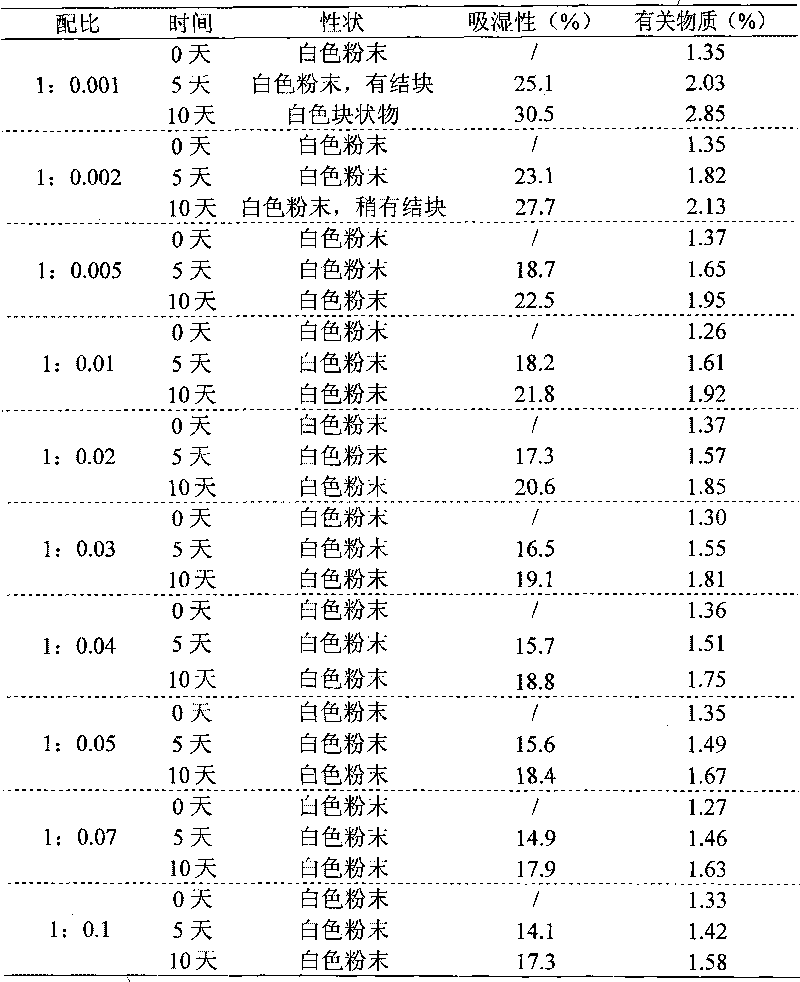
Drug composition of meclofenoxate hydrochloride and polyethylene glycol and preparation method thereof
InactiveCN101756952AImprove securityImprove stabilityOrganic active ingredientsNervous disorderPolyethylene glycolMeclofenoxate Hydrochloride
Owner:HAINAN SIHUAN PHARMA +1
Meclofenoxate hydrochloride medicine use
ActiveCN101069683AGood curative effectOrganic active ingredientsSkeletal disorderRight femoral headIntramuscular injection
The present invention relates to a new application of meclofenoxate hydrochloride for preparing medicine for curing necrosis of femoral head with good therapeutic effect. The invented medicine can be made into injection preparation, also can be made into oral preparation, for example meclofenoxate hydrochloride capsule, etc. Said invention also provides its application method.
Owner:珠海晨安医药有限公司
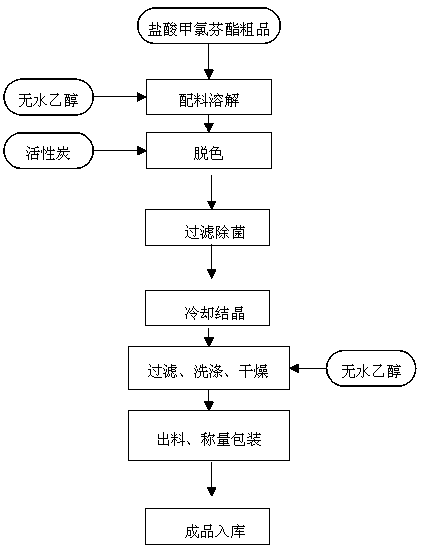
Purification process of meclofenoxate hydrochloride
InactiveCN104058979AProcess stabilityImprove qualityOrganic compound preparationAmino-hyroxy compound preparationAnhydrous ethanolMoisture
The invention discloses a purification process of meclofenoxate hydrochloride. The purification process of meclofenoxate hydrochloride includes the following steps that before production, detection instruments, production devices and work sites are detected and cleaned, salt shakers are started in a vacuum state, anhydrous ethanol is sucked into the salt shakers, crude meclofenoxate hydrochloride products are stirred and dissolve in the anhydrous ethanol, medicinal carbon is put into the salt shakers and mixed for decoloration, feed liquid is sterilized and filtered from the salt shakers level by level, input in a crystallization tank, cooled, crystallized and filtered, vacuum drying is conducted after two washing processes are finished, and cooling and discharge can be conducted after it is detected that moisture in crystals is below 0.5%. The purification process is stable, meclofenoxate hydrochloride with high quality and purity can be purified through the crude meclofenoxate hydrochloride products, and the yield can reach 80.0% to 88.0%.
Owner:JIANGSU HI STONE PHARMA
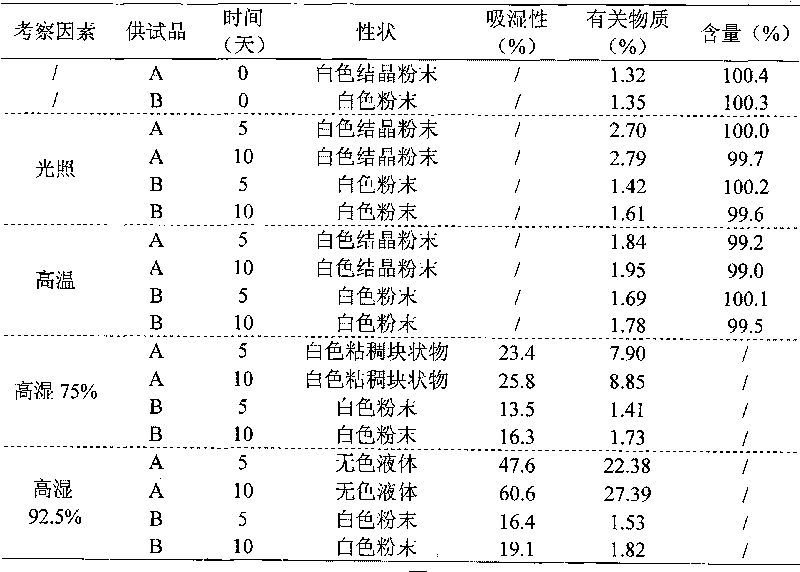
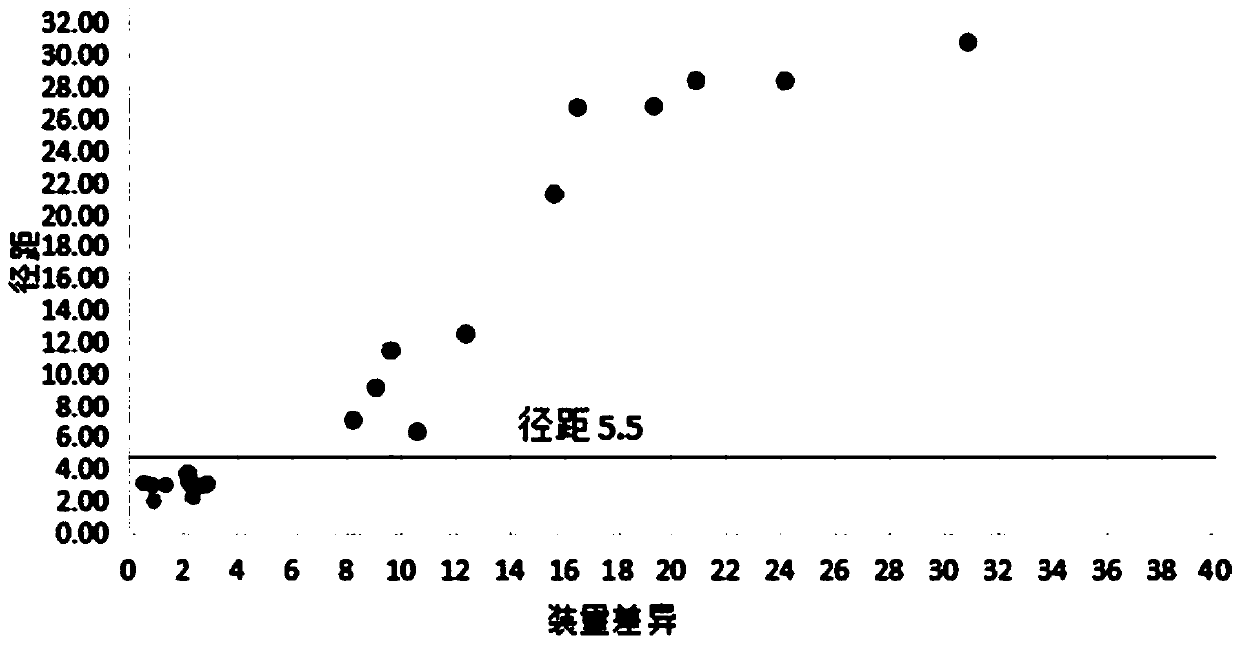
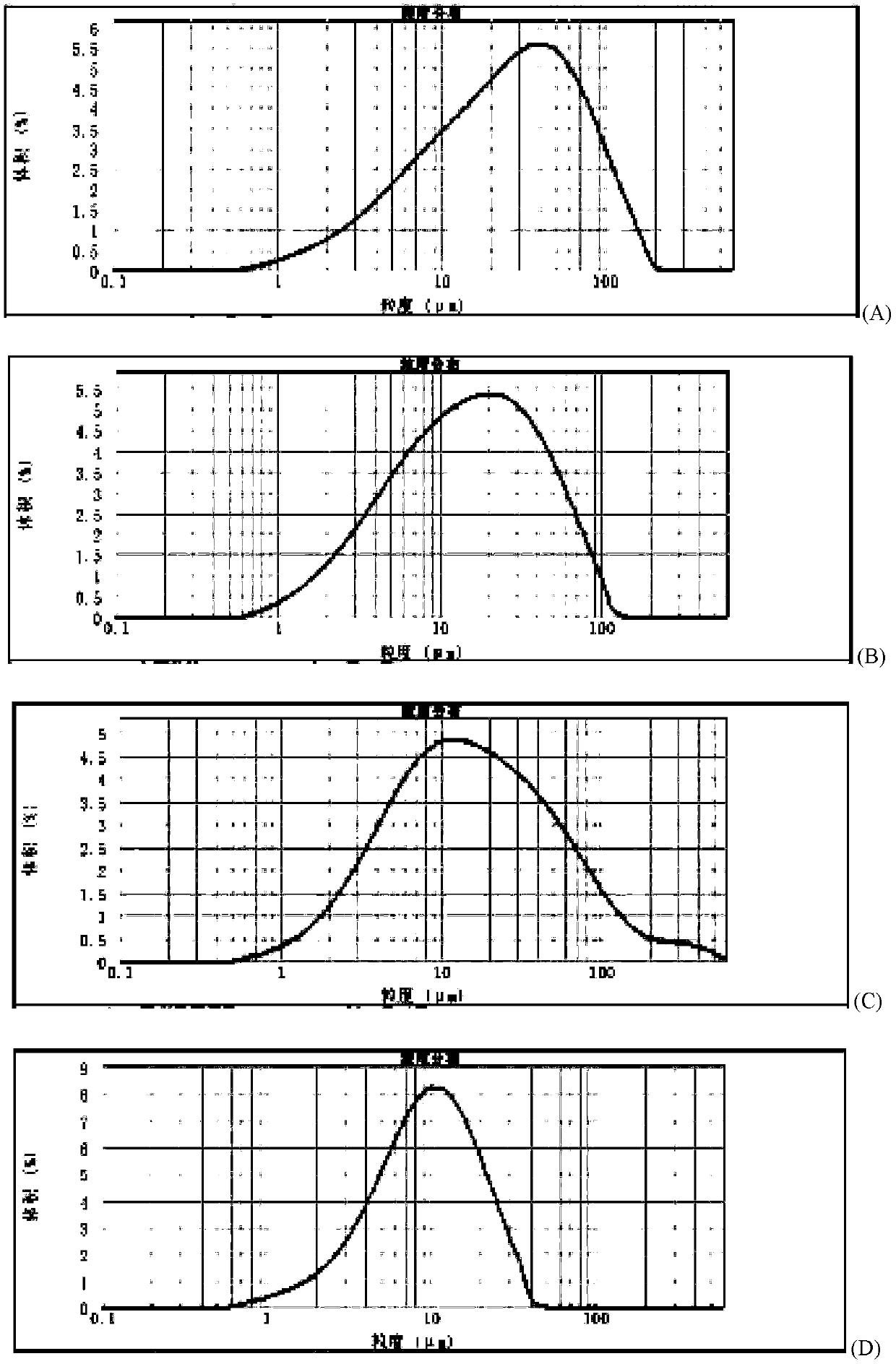
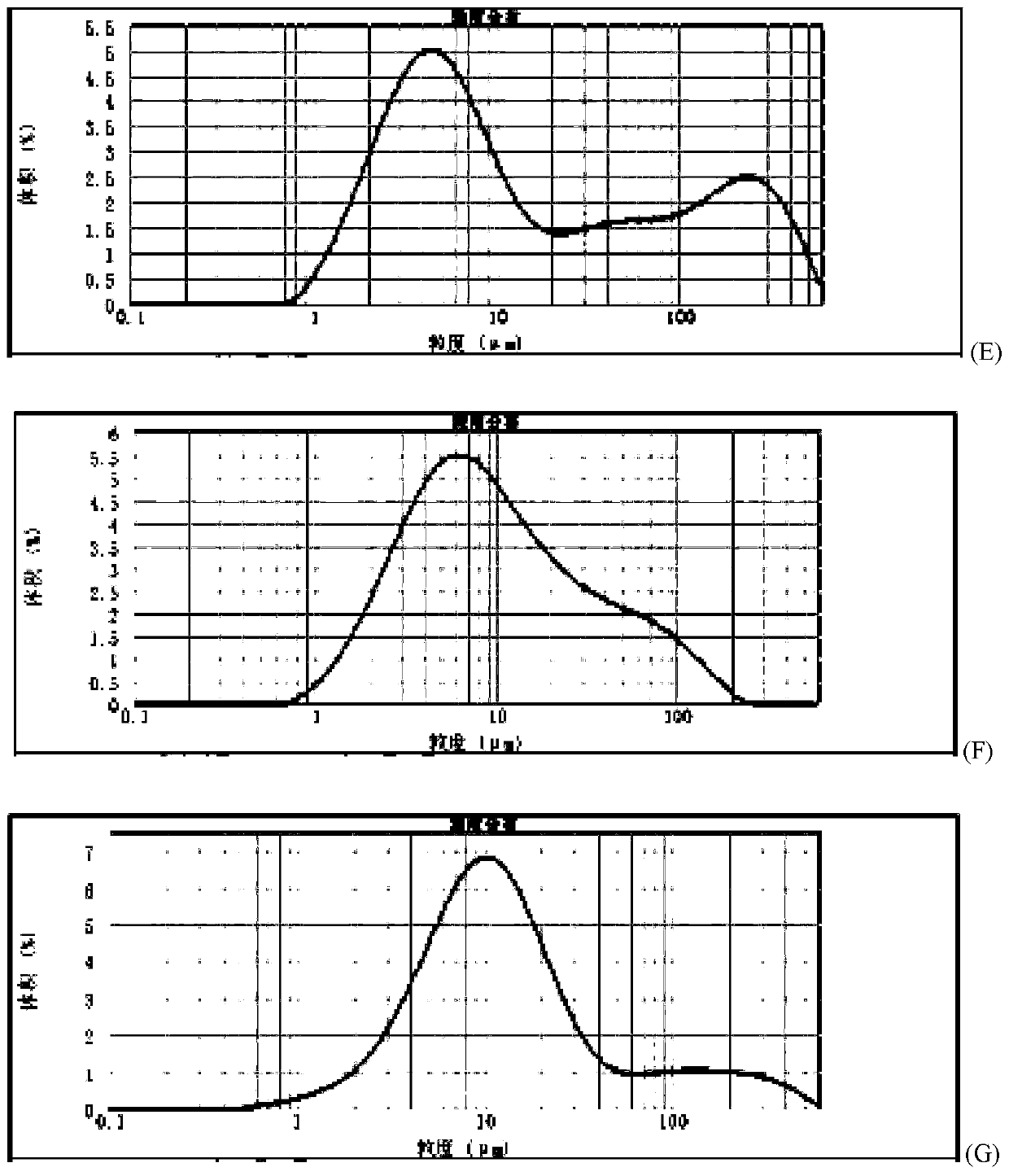
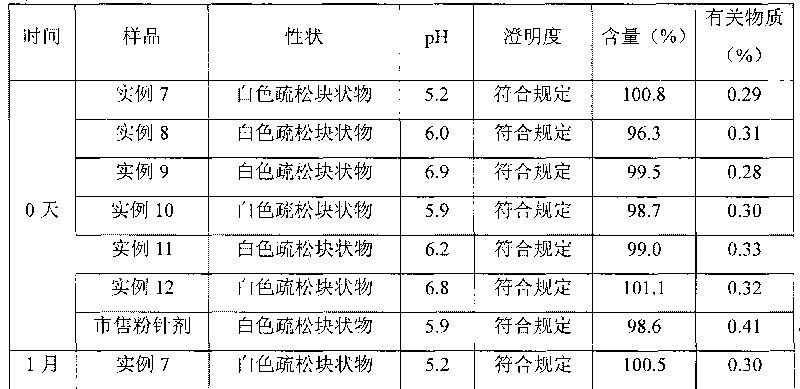
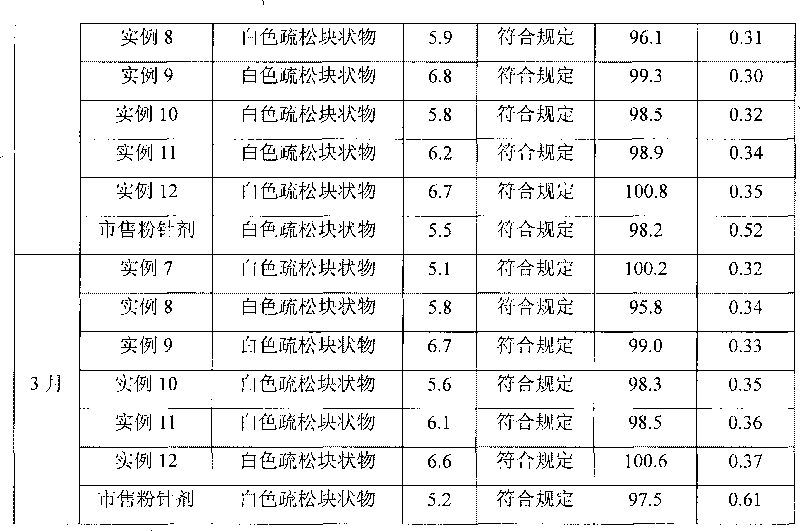
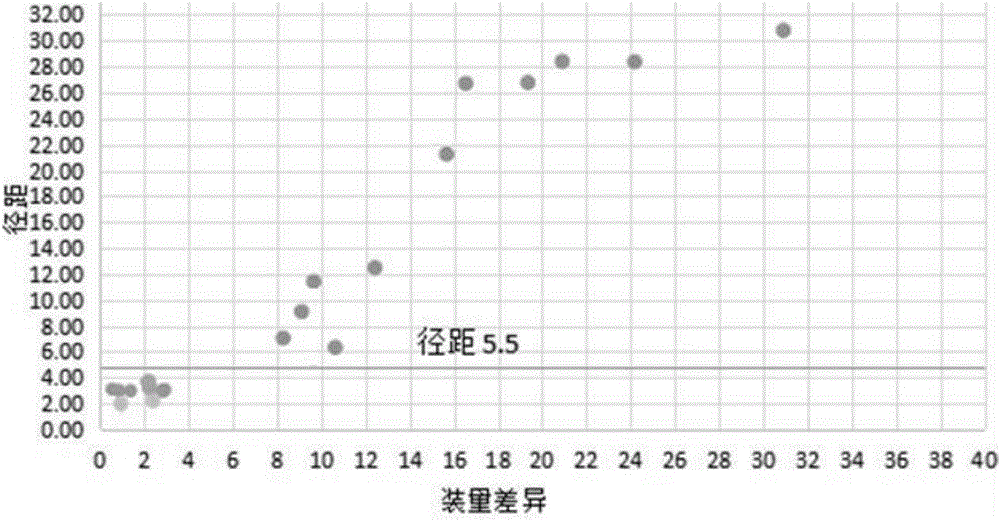
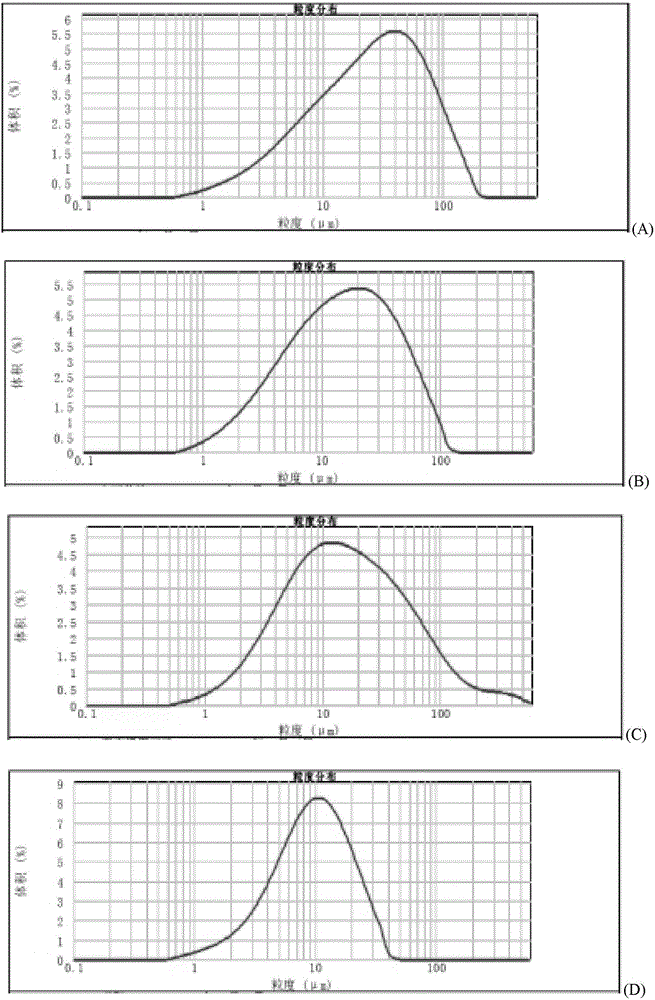
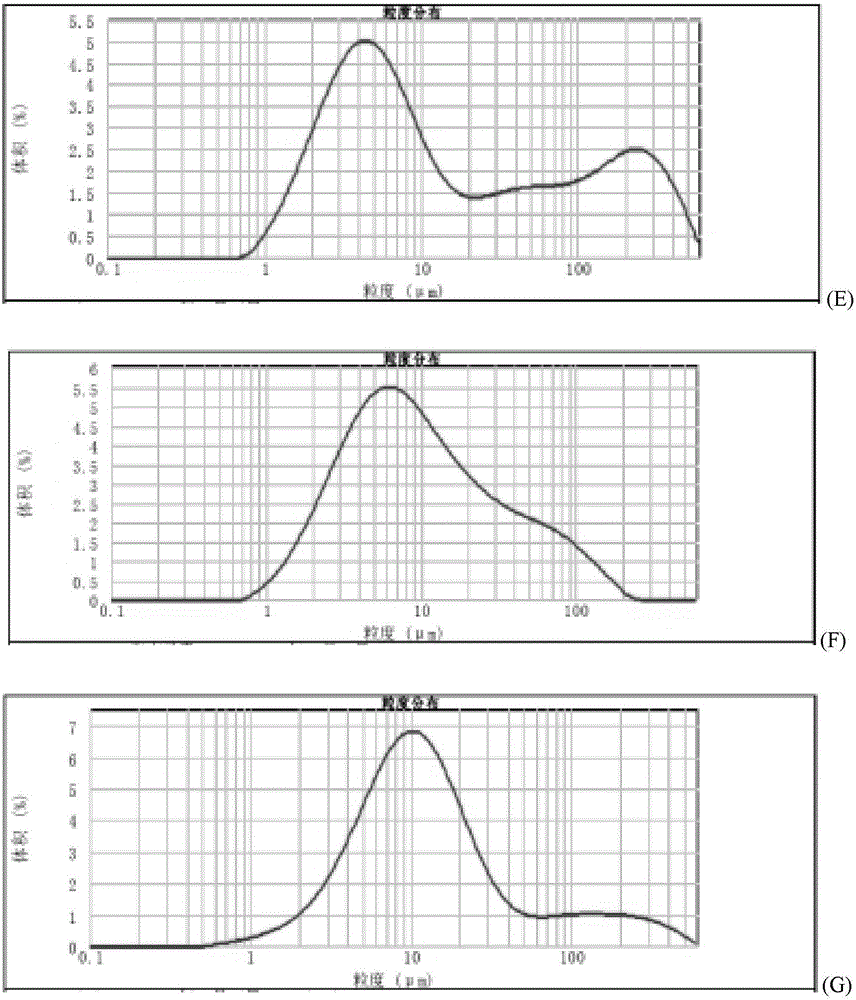
Method for quickly judging the quality status of meclofenoxate hydrochloride powder injection
The invention belongs to the technical field of medicines, and particularly relates to a method for quickly judging the quality condition of the meclofenoxate hydrochloride powder injection. The method comprises the following steps of (1) providing a meclofenoxate hydrochloride powder injection test sample; (2) determining the particle size and the particle size distribution of the test sample by using a laser scattering granularity distribution instrument and a light scattering method, and calculating Dv10, Dv50 and Dv90 values of test sample particles; (3) calculating the span of the test sample powder by the following formula: Span=(Dv90-Dv10) / Dv50; and (4) determining the quality condition of the meclofenoxate hydrochloride powder injection according to the result of the span value. The invention further relates to a method for preparing the meclofenoxate hydrochloride powder injection by virtue of the span parameter value. According to the method provided by the invention, the quality condition of the meclofenoxate hydrochloride powder injection can be quickly judged, and the obtained product has excellent property when the meclofenoxate hydrochloride powder injection is prepared by virtue of the method.
Owner:BEIJING INST FOR DRUG CONTROL
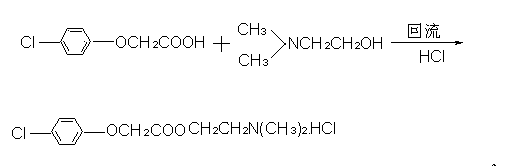
Preparation method of meclofenoxate hydrochloride
ActiveCN109400489ALow reaction temperatureHigh reaction yieldOrganic compound preparationCarboxylic compound preparationReaction temperatureP-chlorophenoxyacetic acid
The invention discloses a preparation method of meclofenoxate hydrochloride. The preparation method comprises the following steps: (A), performing an acylating chlorination reaction on p-chlorophenoxyacetic acid and sulfoxide chloride to obtain p-chlorophenoxyacetyl chloride; (B), performing a condensation reaction on the p-chlorophenoxyacetyl chloride obtained in the step (A) and dimethylaminoethanol to obtain meclofenoxate free alkali; (C), producing the free alkali obtained in the step (B) into hydrochloride, preserving heat and filtering to obtain the meclofenoxate hydrochloride. By the preparation method, raw materials are easy to obtain, the reaction temperature is low, the operation is convenient, and a crude product is high in purity and stable in quality; the invention provides asynthesis route of the meclofenoxate hydrochloride, which is easier to operate, more environmentally friendly and more economical.
Owner:GUANGDONG HUANAN PHARMACEUTICAL GROUP CO LTD +1
Pharmaceutical meclofenoxate hydrochloride composition for treating pediatric enuresis
InactiveCN105213361AImprove liquidityReduce moistureOrganic active ingredientsOrganic chemistrySide reactionBiology
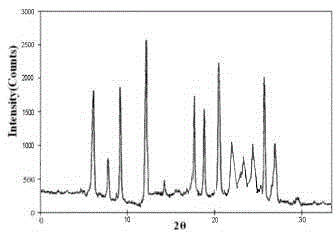
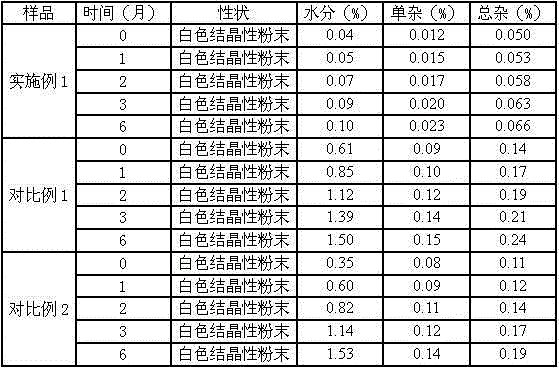
The invention discloses a pharmaceutical meclofenoxate hydrochloride composition for treating pediatric enuresis and belongs to the technical field of medicines. The pharmaceutical meclofenoxate hydrochloride composition consists of meclofenoxate hydrochloride and sodium chloride, wherein meclofenoxate hydrochloride is in the form of crystals, and an X-ray powder diffraction pattern obtained through Cu-K alpha ray measurement is as shown in the Figure 1. The novel crystal form of meclofenoxate hydrochloride, provided by the invention, is different from the crystal form structure in the prior art. Tests verify that the provided meclofenoxate hydrochloride composition is remarkably improved in hygroscopicity and fluidity, extremely low in moisture and impurity content and better in stability than the prior art, and provides convenience for agent preparation. The meclofenoxate hydrochloride composition provided by the invention is higher in bioavailability and lower in side reaction rate than the prior art. A powder injection prepared from the novel crystal form compound is excellent in fluidity, extremely low in moisture and impurity content, better in stability, high in bioavailability and low in side reaction rate.
Owner:杨献美
Central stimulant meclofenoxate hydrochloride composition
InactiveCN105106190AImprove liquidityReduce moistureOrganic active ingredientsPowder deliveryMoisture absorptionBioavailability
The invention relates to a central stimulant meclofenoxate hydrochloride composition and belongs to the technical field of medicines. The composition is prepared from meclofenoxate hydrochloride and anhydrous sodium carbonate, wherein the meclofenoxate hydrochloride is crystal and an X-ray powder diffraction pattern obtained through measurement by using Cu-K alpha rays is as shown in picture 1. The new crystal form of the meclofenoxate hydrochloride provided by the invention is different from the crystal form structures in the prior art. As proved by tests, compared with the prior art, the meclofenoxate hydrochloride crystal compound provided by the invention has the advantages that the moisture absorption and flowability are obviously improved, the content of water and impurities is extremely low, the stability is better and convenience is provided for the preparation of preparations. Compared with the prior art, the meclofenoxate hydrochloride crystal compound provided by the invention has higher bioavailability and the side reaction rate is reduced. Compared with the prior art, powder-injection prepared by using the compound of the new crystal form has the advantages of good flowability, extremely low content of water and impurities, better stability, high bioavailability and low side reaction rate.
Owner:杨献美
Drug meclofenoxate hydrochloride compound for adjusting nerve cell metabolism
InactiveCN105130828AGood hygroscopicityReduce moistureOrganic compound preparationAmino-hyroxy compound preparationMeclofenoxatePharmaceutical drug
The invention relates to a drug meclofenoxate hydrochloride compound for adjusting nerve cell metabolism, belonging to the technical field of medicine. An x-ray powder diffraction pattern obtained by Cu-Kalpha ray measurement on the meclofenoxate hydrochloride compound is shown as picture 1. Compared with the prior art, the meclofenoxate hydrochloride crystal form compound is obviously improved in hygroscopicity and fluidity, extremely low in moisture and impurity content and better in stability, provides convenience for preparing a preparation, and is high in bioavailability and lower in side reaction rate.
Owner:QINGDAO HUAZHICAO PHARMA CO LTD
Meclofenoxate hydrochloride medicinal composition and method for preparing freeze-drying powder injection of meclofenoxate hydrochloride medicinal composition
InactiveCN102552234AAvoid hydrolysisImprove stabilityOrganic active ingredientsPowder deliverySide effectMANNITOL/SORBITOL
Owner:湖北安邦医药有限公司
Brain neurotrophic drug meclofenoxate hydrochloride composition
InactiveCN105343046AImprove liquidityReduce moisturePowder deliveryOrganic active ingredientsMeclofenoxateArginine
The invention belongs to the technical field of medicine and relates to a brain neurotrophic drug meclofenoxate hydrochloride composition, comprising meclofenoxate hydrochloride and arginine. The meclofenoxate hydrochloride is a crystal, its X-ray powder diffraction pattern measured using Cu-KAlpha ray is shown as in Figure 1. The novel crystal form of the meclofenoxate hydrochloride is different from prior art crystal form structures, experiments show that the meclofenoxate hydrochloride crystal form compound has significantly improved moisture absorption and fluidity when compared to the prior art, the moisture and impurity content is very low, the stability is better, and the preparation of preparations is facilitated. Compared with the prior art, the meclofenoxate hydrochloride crystal form compound is higher in bioavailability and lower in side effect rate. Compared with the prior art, a powder injection made with the novel crystal from compound has good fluidity, very low moisture and impurity content, better stability, high bioavailability and low side effect rate.
Owner:杨献美
Application of meclofenoxate hydrochloride in preparation of medicine for preventing or treating Parkinson's disease
PendingCN113332271APrevent synaptic damagePrevent diseaseOrganic active ingredientsNervous disorderPharmaceutical SubstancesSynexpression
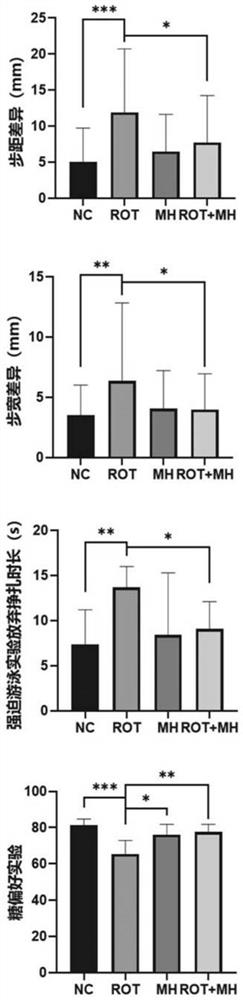
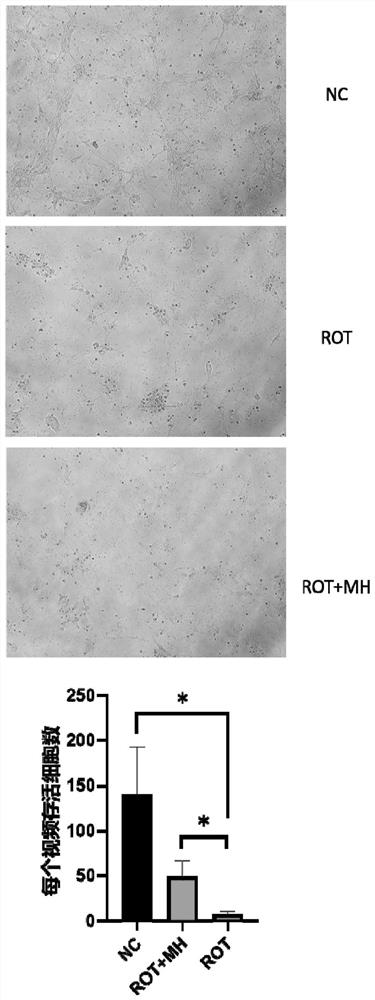
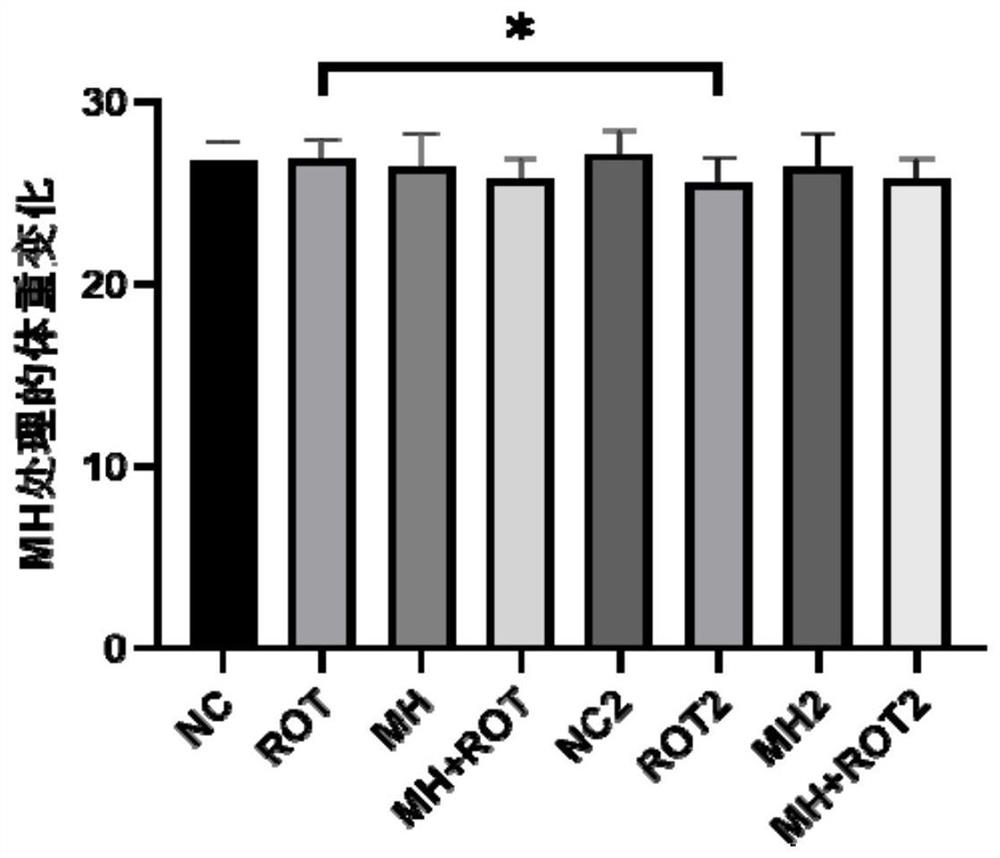
The invention relates to the field of biological pharmacy, in particular to application of meclofenoxate hydrochloride (MH) in preparation of a medicine for preventing or treating Parkinson's disease. By integrating multi-omics data to explore a gene co-expression network and applying cell and animal experiments to verify, the meclofenoxate hydrochloride can obviously prevent synaptic injury caused by mitochondrial function decline in the Parkinson's disease, can effectively reduce mitochondrial membrane injury and reduce mitochondrial edema, and can effectively enable the expression of synapsis specific proteins to rise; it is indicated that the Parkinson's disease characterized by neuronal synaptic lesion can be prevented, dopamine activity is improved, Parkinson's disease lesion is prevented, and behavioral symptoms of the Parkinson's disease is improved.
Owner:SUN YAT SEN MEMORIAL HOSPITAL SUN YAT SEN UNIV
Meclofenoxate hydrochloride dry suspension and preparation method thereof
InactiveCN103479580AGreat tasteEasy to useOrganic active ingredientsPowder deliveryAlcoholismsNeonatal anoxia
The invention belongs to the technical field of medicines, and relates to a meclofenoxate hydrochloride dry suspension and a preparation method thereof. The meclofenoxate hydrochloride dry suspension is used for treating traumatic coma, alcoholism, neonatal anoxia and child enuresis. The meclofenoxate hydrochloride dry suspension has a relatively-good taste, is simple and feasible in preparation process, and is more suitable for old patients and child patients when compared with the preparation products on sale at present.
Owner:天津市聚星康华医药科技有限公司
Meclofenoxate hydrochloride medicinal composition and method for preparing freeze-drying powder injection of meclofenoxate hydrochloride medicinal composition
InactiveCN102552234BAvoid hydrolysisImprove stabilityOrganic active ingredientsPowder deliveryFreeze-dryingMannitol
The invention provides a meclofenoxate hydrochloride medicinal composition and a method for preparing a freeze-drying powder injection of the meclofenoxate hydrochloride medicinal composition. The meclofenoxate hydrochloride medicinal composition comprises the following components in part by weight: 30 to 100 parts of meclofenoxate hydrochloride, 60 to 120 parts of reduced glutathione and 40 to 80 parts of mannitol. The preparation method comprises the following steps of: a) dissolving the mannitol in water for injection, adding the meclofenoxate hydrochloride, and regulating the pH value to be 2-5; b) adding the reduced glutathione, stirring uniformly, adsorbing the solution by using 0.01 percent active carbon and filtering; and c) performing freeze drying to obtain the freeze-drying powder injection. The meclofenoxate hydrochloride medicinal composition has a reasonable formula and a good curative effect, and is high in quality stability, small in side effects and high in safety, and the adverse reaction of patients on medicine is reduced. The method for preparing the freeze-drying powder injection of the meclofenoxate hydrochloride medicinal composition is reasonable, reliable, simple, feasible, and suitable for industrial production.
Owner:湖北安邦医药有限公司
Applicaiton of meclofenoxate hydrochloride in preparing medicine for treating femoral head necrosis
ActiveCN100515417CGood curative effectOrganic active ingredientsSkeletal disorderVeinOral medication
The invention relates to the medical use of meclofenoxate hydrochloride. The present invention has good curative effect in treating femoral head necrosis with meclofenoxate hydrochloride. The medicine of the present invention (such as meclofenoxate hydrochloride for injection) can be given by intramuscular injection, intravenous injection or intravenous drip, and can also be given orally by oral preparations (such as meclofenoxate hydrochloride capsules, etc.). Its dosage can generally be administered according to the conventional dosage of meclofenoxate hydrochloride for injection in the prior art. Recommended usage and dosage of the present invention: intravenous injection or intravenous drip, 2-3 times a day, 0.06-0.25g each time; intramuscular injection, 0.06-0.25 once, once every 2 hours.
Owner:珠海晨安医药有限公司
Meclofenoxate hydrochloride stomach-floating sustained release capsule and preparing method thereof
InactiveCN101229149BImprove stabilityRelease stabilityOrganic active ingredientsNervous disorderSustained Release CapsuleCurative effect
The invention discloses a meclofenoxate hydrochloride intra-gastric floating sustained release capsule. The invention adopts the intra-gastric floating sustained release technique to prevent the bad effect to the stability of the medicine in capsules caused by the elevation of the PH value in gastrointestinal tract, avoid intestinal juice in human body from retrograding the meclofenoxate hydrochloride in capsules, which ensures that the medicine can release steadily, slowly and completely. The capsule of the invention should be taken twice every day and can float continuously in stomach for more than 8 hours; the medicine can be released for 12 hours continuously and the release rate at last reaches to over 90 percent, thereby enhancing the stability of the medicine, prolonging the curingeffect, greatly being convenient for patients to take medicine. The invention also provides the preparation method.
Owner:SHANGHAI PHARMA IND
The synthetic technique of meclofenoxate hydrochloride crude product
ActiveCN104072382BImprove qualityReduce manufacturing costOrganic compound preparationAmino-hyroxy compound preparationRefluxFiltration
The invention discloses a synthetic process of crude meclofenoxate hydrochloride. The synthetic process comprises the following steps: checking and cleaning a detection instrument, production equipment and a working place before production, performing a heating reflux reaction on toluene, tolueneaminoethanol, p-chlorophenoxyacetic acid and toluenesulfonic acid, cooling, decolorizing, performing suction filtration on decolorizing liquid, standing and layering filter liquor, performing suction filtration, immersion cleaning and vacuum drying on upper-layer crystals to prepare crude meclofenoxate hydrochloride, and finally weighing and packaging. According to the synthetic process, high-quality crude meclofenoxate hydrochloride can be prepared, the yield is 74.0%-78.0%, a good foundation is laid for refining meclofenoxate hydrochloride, and the production cost is reduced.
Owner:JIANGSU HI STONE PHARMA
Orally disintegrating tablet containing meclofenoxate hydrochloride and preparation method thereof
InactiveCN103494784AGreat tasteSimple manufacturing processOrganic active ingredientsNervous disorderAlcoholismsOrally disintegrating tablet
The invention belongs to the technical field of medicine and relates to an orally disintegrating tablet containing meclofenoxate hydrochloride and a preparation method thereof. The orally disintegrating tablet containing meclofenoxate hydrochloride is used for treating traumatic coma, alcoholism, neonatal anoxia and nocturia enuresis. The orally disintegrating tablet containing meclofenoxate hydrochloride tastes good and the preparation technology is simple and feasible. Compared with products available in the market at present, the orally disintegrating tablet containing meclofenoxate hydrochloride is quite suitable for the old, children and patients with dysphagia.
Owner:天津市聚星康华医药科技有限公司
Preparation method of meclofenoxate hydrochloride freeze-dried powder injection
PendingCN113133975ALow hygroscopicityReduce moisture absorption performancePowder deliveryOrganic active ingredientsMeclofenoxateActivated carbon
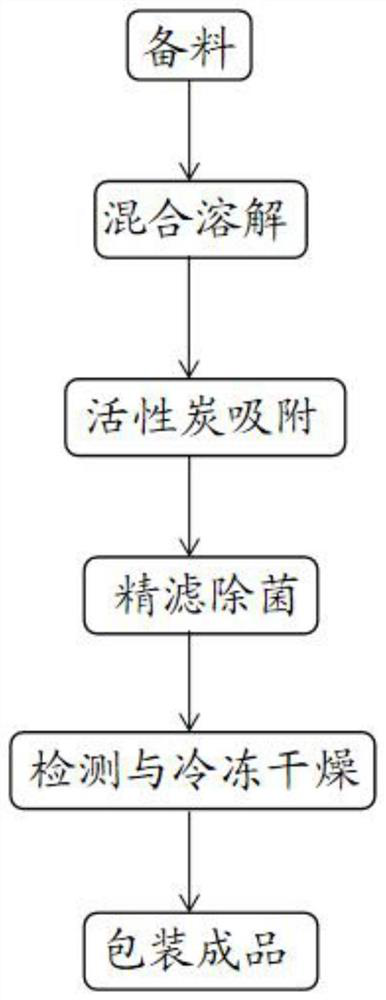
The invention discloses a preparation method of a meclofenoxate hydrochloride freeze-dried powder injection. The preparation method comprises the following six steps: preparing materials, mixing and dissolving, adsorbing with activated carbon, finely filtering and sterilizing, detecting and freeze-drying, and packaging to obtain a finished product. A used prescription comprises the following components in parts by weight: 2 to 4 parts of meclofenoxate hydrochloride, 0.01 to 0.5 part of polyethylene glycol and 4 to 14 parts of excipient. The excipient comprises mannitol, and the prescription dosage of the mannitol is 45% to 70% of the prescription dosage of the meclofenoxate hydrochloride. According to the preparation method provided by the invention, the prepared meclofenoxate hydrochloride freeze-dried powder injection is good in moisture-resistant effect and good in medicine stability, and the technical problems that an existing meclofenoxate hydrochloride injection preparation is poor in moisture resistance, and the medicine stability is prone to being damaged are solved.
Owner:CHINA MEHECO SANYANG PHARMA CO LTD
Meclofenoxate hydrochloride pharmaceutical composition capable of promoting brain energy metabolism
InactiveCN105106189AImprove liquidityReduce moistureOrganic active ingredientsNervous disorderMeclofenoxatePharmaceutical drug
The invention discloses a meclofenoxate hydrochloride pharmaceutical composition capable of promoting brain energy metabolism, and belongs to the technical field of medicines. The composition is prepared from meclofenoxate hydrochloride and sodium dihydrogen phosphate, wherein the meclofenoxate hydrochloride is crystal, and an X-ray powder diffraction pattern obtained by measuring through using a Cu-K alpha ray is shown as a figure 1. The novel crystal form of the meclofenoxate hydrochloride provided by the invention is different from the structure of an existing crystal from in the prior art. Through experimental verification, a meclofenoxate hydrochloride crystal compound provided by the invention is greatly improved in hygroscopicity and fluidity in comparison with those in the prior art, the water content and the impurity content are extremely low, the stability is good, and convenience is brought for the preparation of a preparation.
Owner:杨献美
Meclofenoxate hydrochloride microcapsule and method for preparing injection thereof
InactiveCN101278924BSimple processUniform particle sizeOrganic active ingredientsNervous disorderAdjuvantFreeze-drying
The invention provides a meclofenoxate hydrochloride microcapsule for injection and a production method thereof. The meclofenoxate hydrochloride microcapsule for the injection is composed of the meclofenoxate hydrochloride and adjuvant, which is characterized in that the adjuvant contains gelatin, dextran and emulsifier. The invention also provides the production method of a meclofenoxate hydrochloride freeze-dry powder and injection. As the meclofenoxate hydrochloride microcapsule with high stability in water is used, the meclofenoxate hydrochloride is not hydrolyzed when redissolving; the clarity is good after redissolving; thereby a product in the invention has the advantages of good stability and good quality, which is good for storing the product for a long time.
Owner:HAINAN LINGKANG PHARMA CO LTD
Method for quickly judging quality condition of meclofenoxate hydrochloride powder injection
ActiveCN106769710AFully understand drug content differencesFully understand the qualityParticle size analysisTest sampleGranularity
The invention belongs to the technical field of medicines, and particularly relates to a method for quickly judging the quality condition of the meclofenoxate hydrochloride powder injection. The method comprises the following steps of (1) providing a meclofenoxate hydrochloride powder injection test sample; (2) determining the particle size and the particle size distribution of the test sample by using a laser scattering granularity distribution instrument and a light scattering method, and calculating Dv10, Dv50 and Dv90 values of test sample particles; (3) calculating the span of the test sample powder by the following formula: Span=(Dv90-Dv10) / Dv50; and (4) determining the quality condition of the meclofenoxate hydrochloride powder injection according to the result of the span value. The invention further relates to a method for preparing the meclofenoxate hydrochloride powder injection by virtue of the span parameter value. According to the method provided by the invention, the quality condition of the meclofenoxate hydrochloride powder injection can be quickly judged, and the obtained product has excellent property when the meclofenoxate hydrochloride powder injection is prepared by virtue of the method.
Owner:BEIJING INST FOR DRUG CONTROL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com