Preparation method of meclofenoxate hydrochloride freeze-dried powder injection
A technology of meclofenxetil hydrochloride and freeze-dried powder injection is applied in the field of cerebral function rehabilitation drugs, which can solve the problems of drug stability destruction, poor moisture resistance, etc., achieve stable drug effect, reduce hygroscopicity or deliquescence, improve The effect of anti-moisture effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
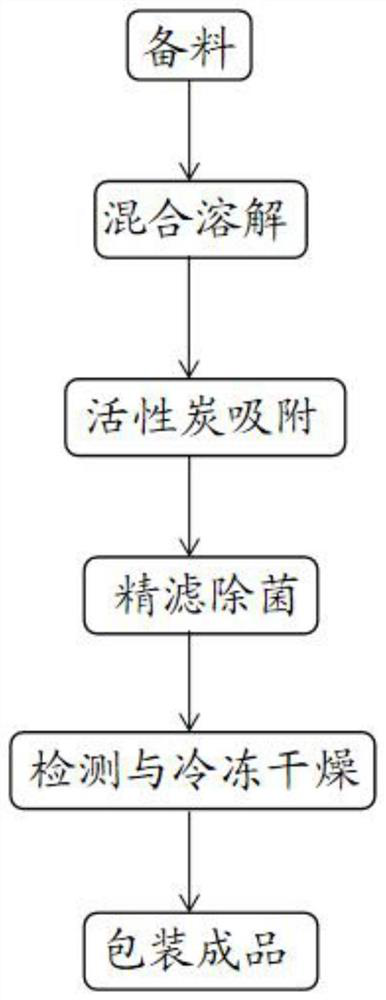
[0030] For ease of understanding, see figure 1 The present application provides an embodiment of a method for preparing a hydrochloride ester frozen, including the following steps:
[0031] Step 1, the preparation: The drug prescription includes 2-4 parts by weight of hydrochloride, 0.01-0.5 parts by weight of polyethylene glycol, 4-14 parts by weight of excipients, including a prescription amount 45% -70% mannitol at the hydrochloride of methoxyl hydrochloride;
[0032] Step 2, mixed dissolution: mix hydrochloride, polyethylene glycol, and excipients to dissolve, during dissolution, water can be added to the drug to dissolve or rate the acid, and stirred for 10-30 minutes after dissolving for 10-30 minutes. 3.0-5.5 mixture;
[0033] Step 3, activated carbon adsorption: Adding a mixture obtained by activated carbon to step 2, stirring adsorption of 10-30 minutes after detonating carbon, preparing a carbon filtrate;
[0034] Step 4, fine filtration and sterilization: The anti-carbo...
Embodiment 2
[0040]As a further improvement of Example 1, the present application provides an embodiment of a method for preparing a hydrochloride ester freeze-dry powder injection, optionally, polyethylene glycol selects PEG-4000. Alternatively, polyethylene glycol is selected from PEG-6000. Alternatively, the addition of activated carbon is from 0.05% to 0.15% by the weight of the mixed liquid. Alternatively, in step 4, a microporous filtrate was sequentially filtered and sterilized. Alternatively, in step 4, a cylindrical microporous filter with a 0.22 μm filter element is sequentially employed is filtered and sterilized. Alternatively, the freeze drying in step 5 includes the following steps: pre-freeze: the filling of the pressed half-plus is placed in a freeze-dried machine having a temperature of -35 ° C to -25 ° C, frozen 4-4.5 hours. Solid-state drug; sublimation: at a constant temperature rise to -15 ° C, the vacuum treatment of the pre-free solid drug is carried out, so that the moi...
Embodiment 3
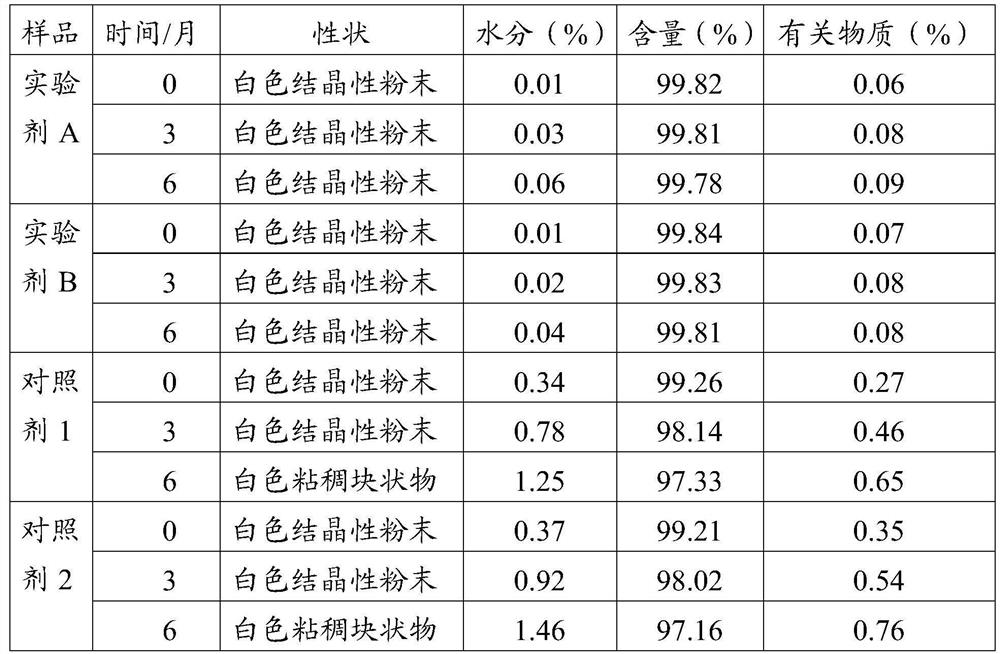
[0045] In order to facilitate understanding of the above embodiments, the present application provides a test description, and the control agent uses two hydrochloride-free frozen powder injections on the market, and labeled a control agent 1 and 2, respectively. In one embodiment, the lyophilized powder needle experimental agent A and B, which can be prepared according to the drug prescription and the present application, and two experimental agents include: 3 parts by weight of hydrochloride, 10 The weight of the excipient, wherein the excipient comprises mannitol, malic acid and trehalose, step 2 (mixed dissolved), the pH of the mixed liquid obtained is 4.3, and the addition of activated carbon is mixed with the mixture. The weight of 0.10% in turn, a microporous filter film having a filtration aperture of 0.45 μm, 0.22 μm is used in two copies, and the freeze drying includes the following steps:
[0046] Pre-ferrite: The filling of the pressed half-plus is placed in a lyophili...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com