Method for quickly judging quality condition of meclofenoxate hydrochloride powder injection
A technology for meclofen axetil hydrochloride and powder injection, which is applied in the field of medicine, can solve the problems of unqualified content, difference in loading, unqualified meclofen axetil powder for injection, etc., and achieves the effects of long working time and large amount of data
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0060] Preparation Example 1: Preparation of Meclofenoxate Hydrochloride Powder for Injection
[0061] (1) Provide sterile meclofenoxate hydrochloride bulk drug;
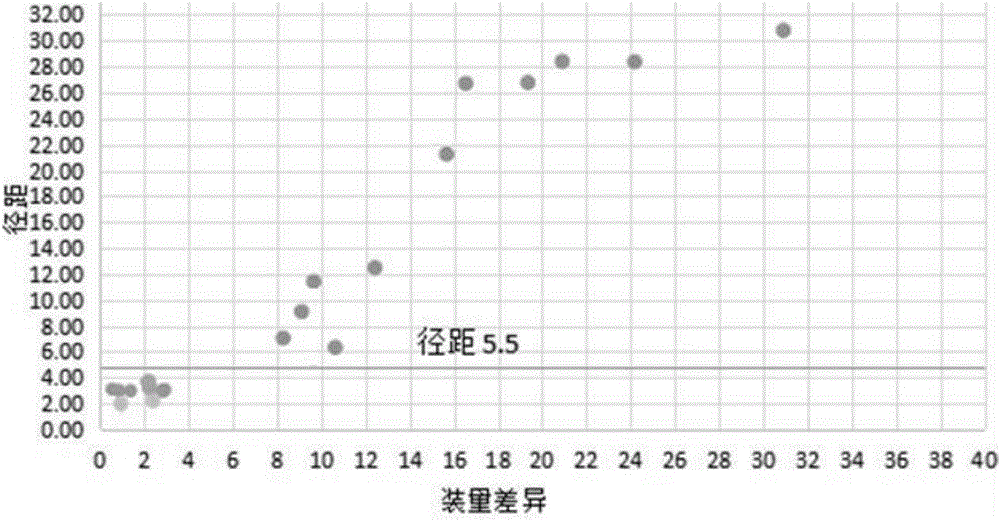
[0062] (2) pulverize the meclofenoxate hydrochloride bulk drug under aseptic conditions, and monitor the diameter span value of the bulk drug particle in real time during the pulverization process;
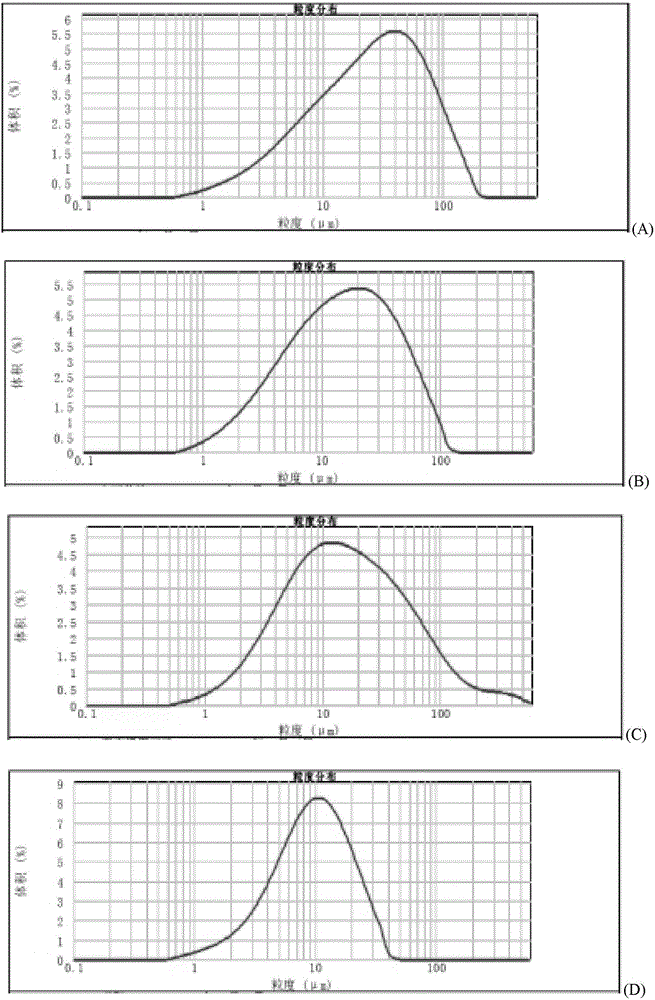
[0063] (3) When the span value of the bulk drug granules is less than 5.5 (3.2 in this example, the Dv50 of the powder is 31um), stop pulverizing;
[0064] (4) Under aseptic conditions, the bulk drug obtained in step (3) is divided into glass bottles (250 mg per bottle), sealed, and obtained.
preparation example 2
[0065] Preparation Example 2: Preparation of Meclofenoxate Hydrochloride Powder for Injection
[0066] (1) Provide sterile meclofenoxate hydrochloride bulk drug;
[0067] (2) pulverize the meclofenoxate hydrochloride bulk drug under aseptic conditions, and monitor the diameter span value of the bulk drug particle in real time during the pulverization process;
[0068] (3) When the span value of the bulk drug granules is less than 5.5 (2.4 in this example, the Dv50 of the powder is 24um), stop pulverizing;
[0069] (4) Under aseptic conditions, the bulk drug obtained in step (3) is divided into glass bottles (100 mg per bottle), sealed, and the product is obtained.
preparation example 3
[0070] Preparation Example 3: Preparation of Meclofenoxate Hydrochloride Powder for Injection
[0071] (1) Provide sterile meclofenoxate hydrochloride bulk drug;
[0072] (2) pulverize the meclofenoxate hydrochloride bulk drug under aseptic conditions, and monitor the diameter span value of the bulk drug particle in real time during the pulverization process;
[0073] (3) When the span value of the bulk drug granule is less than 5.5 (5.3 in this example, the Dv50 of the powder is 37um), stop pulverizing;
[0074] (4) Under aseptic conditions, the bulk drug obtained in step (3) is divided into glass bottles (500 mg per bottle), sealed, and the product is obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com