Meclofenoxate hydrochloride stomach-floating sustained release capsule and preparing method thereof
A technology of meclofen axetil hydrochloride and sustained-release capsules, applied in the field of medicine, can solve the problems of not being able to satisfy various patients, unable to effectively solve the problem of the stability of meclofen axetil hydrochloride, etc., and achieve convenient medication, complete release, and enhanced stability sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1) Prescription composition of ball core (1000 capsules)
[0035] Component name Weight
[0036] Meclofenoxate hydrochloride 50.0g
[0037] Microcrystalline Cellulose 40.0g
[0038] Cetyl Alcohol 300.0g
[0039] Cross-linked polyvinylpyrrolidone 10.0g
[0040] Specification: 400mg / capsule
[0041] 2) Ball core preparation process
[0042] The raw material drug is properly dried and pulverized, and passed through a 100-mesh sieve; another microcrystalline cellulose, cetyl alcohol, and cross-linked polyvinylpyrrolidone are respectively passed through a 80-mesh sieve; Appropriate amount of aqueous solution, made of soft materials, prepared pellets by extrusion-spheronization method, dried at 40°C for 3 hours, granulated with 20-28 mesh sieves, and set aside.
[0043] 3) Prescription of coating solution
[0044] Ethyl cellulose (20cp) 20g
[0045] Macrogol 400 1g
[0047] 80% ethanol 1000mL
[0048] 4) Coating process
[0049] The conventio...
example 1
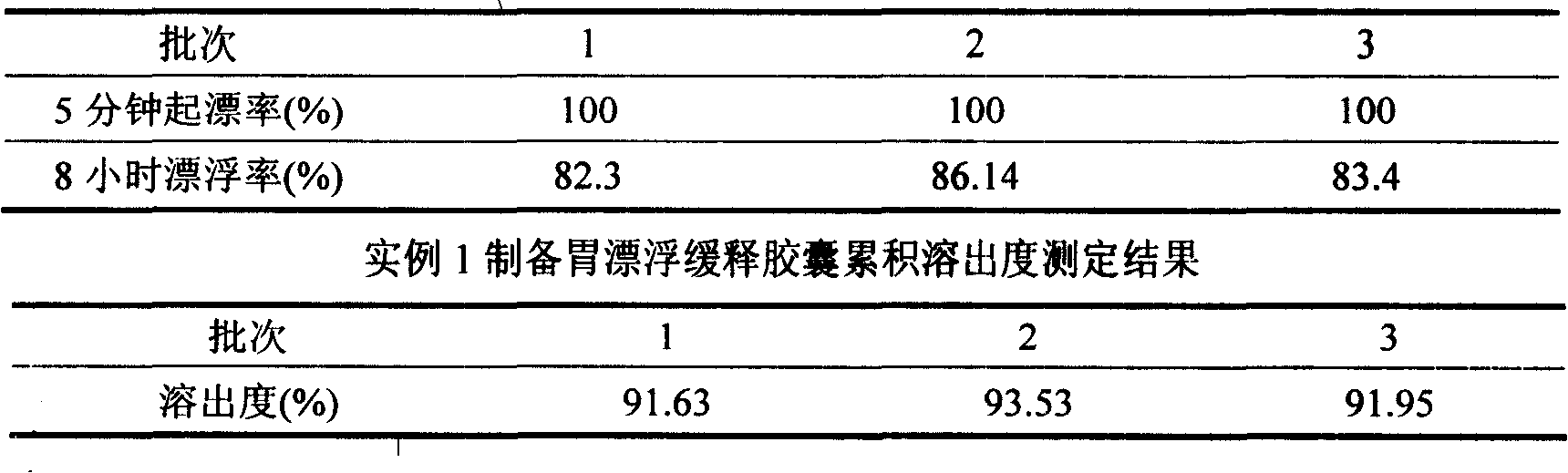
[0051] Example 1 Preparation of Gastric Float Sustained-release Capsules 5-minute Float Rate and 8-Hour Float Rate Investigation
[0052]
Embodiment 2
[0054] 1) Prescription composition of ball core (1000 capsules)
[0055] Component name Weight
[0056] Meclofenoxate hydrochloride 50.0g
[0057] Starch 20.0g
[0058] Microcrystalline Cellulose 20.0g
[0059] Octadecanol 300.0g
[0060] Cross-linked polyvinylpyrrolidone 10.0g
[0061] Specification: 400mg / capsule
[0062] 2) Ball core preparation process
[0063] The raw material drug was properly dried and pulverized, and passed through a 100-mesh sieve; another microcrystalline cellulose, starch, stearyl alcohol, and cross-linked polyvinylpyrrolidone were respectively passed through a 80-mesh sieve; An appropriate amount of aqueous solution of -5 is used to make soft materials, and pellets are prepared by extrusion-spheronization method, dried at 40°C for 3 hours, granulated with a 20-28 mesh sieve, and set aside.
[0064] 3) Prescription of coating solution
[0065] Ethyl cellulose (20cp) 20g
[0066] Macrogol 400 1g
[0068] 80% ethanol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com