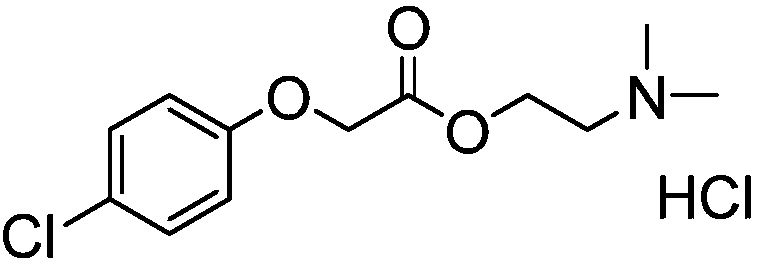
Preparation method of meclofenoxate hydrochloride
A technology of meclofenoxate hydrochloride and hydrochloride, which is applied in the field of preparation of meclofenoxate hydrochloride, can solve problems such as insufficient hydrochloride formation, high reaction temperature, and low product purity, and achieve stable quality and high yield The effect of high and high purity of the crude product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
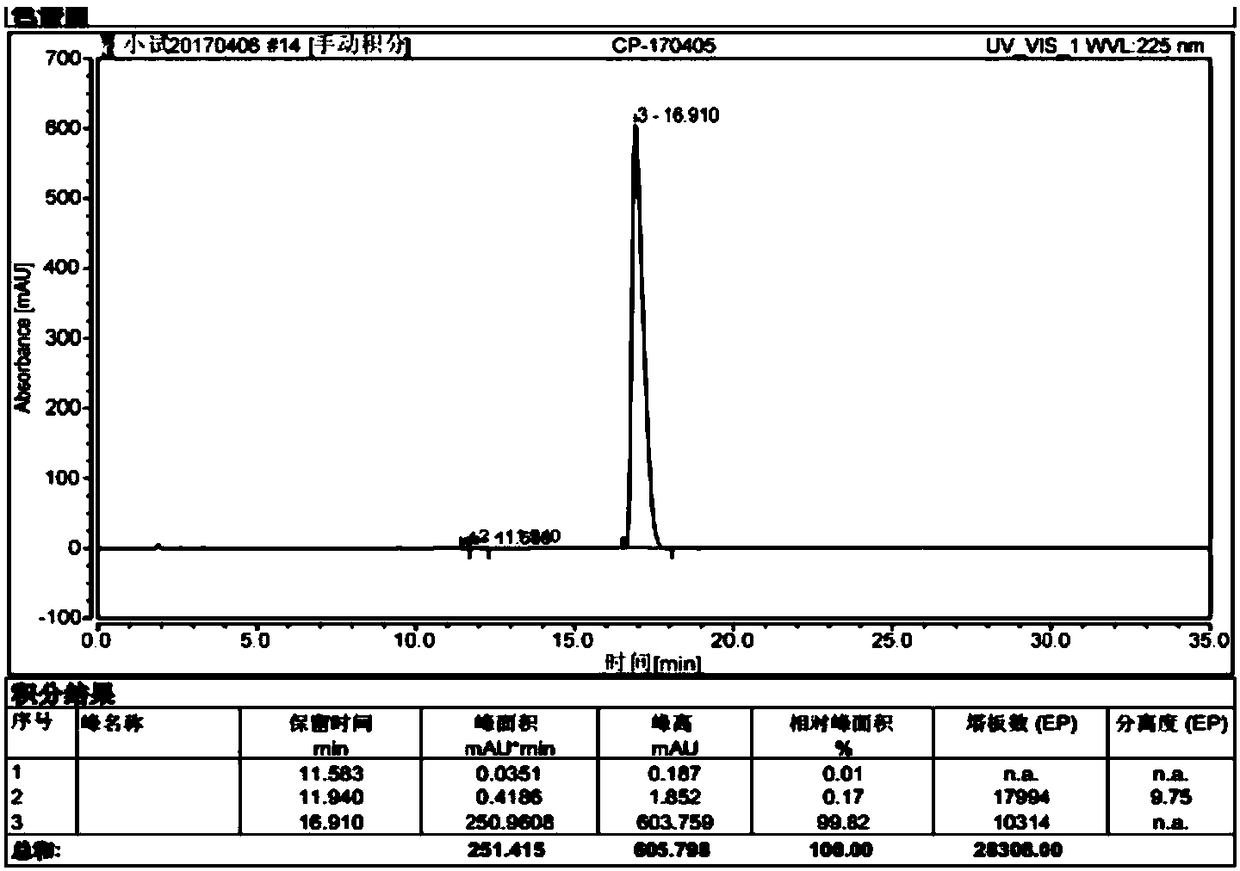
[0038] Put 50 g (0.26 mol) of p-chlorophenoxyacetic acid into dichloromethane in a single-necked bottle, and then add 47.5 g (0.40 mol) of thionyl chloride dropwise. After the dropwise addition, stir and react at 30°C. Concentration of the solvent gave an oil. Dichloromethane was added to the obtained oily substance, and at the same time, 26 g (0.28 mol) of dimethylaminoethanol (0.28 mol) of dichloromethane solution was added dropwise into the reaction flask, and the dropwise addition was completed in 30 minutes, and the reaction was stirred at room temperature. Add water to extract, the obtained organic layer, and the oily substance obtained by concentrating the solvent are added to 250ml of ethyl acetate, and hydrogen chloride gas is used to form hydrochloride until a large amount of solids are precipitated. After being kept at 25°C, it is filtered to obtain 66g of white solid, with a yield of 84.6 %, purity 99.8% (attached figure 1 ):
[0039] The reaction equation is as ...
Embodiment 2
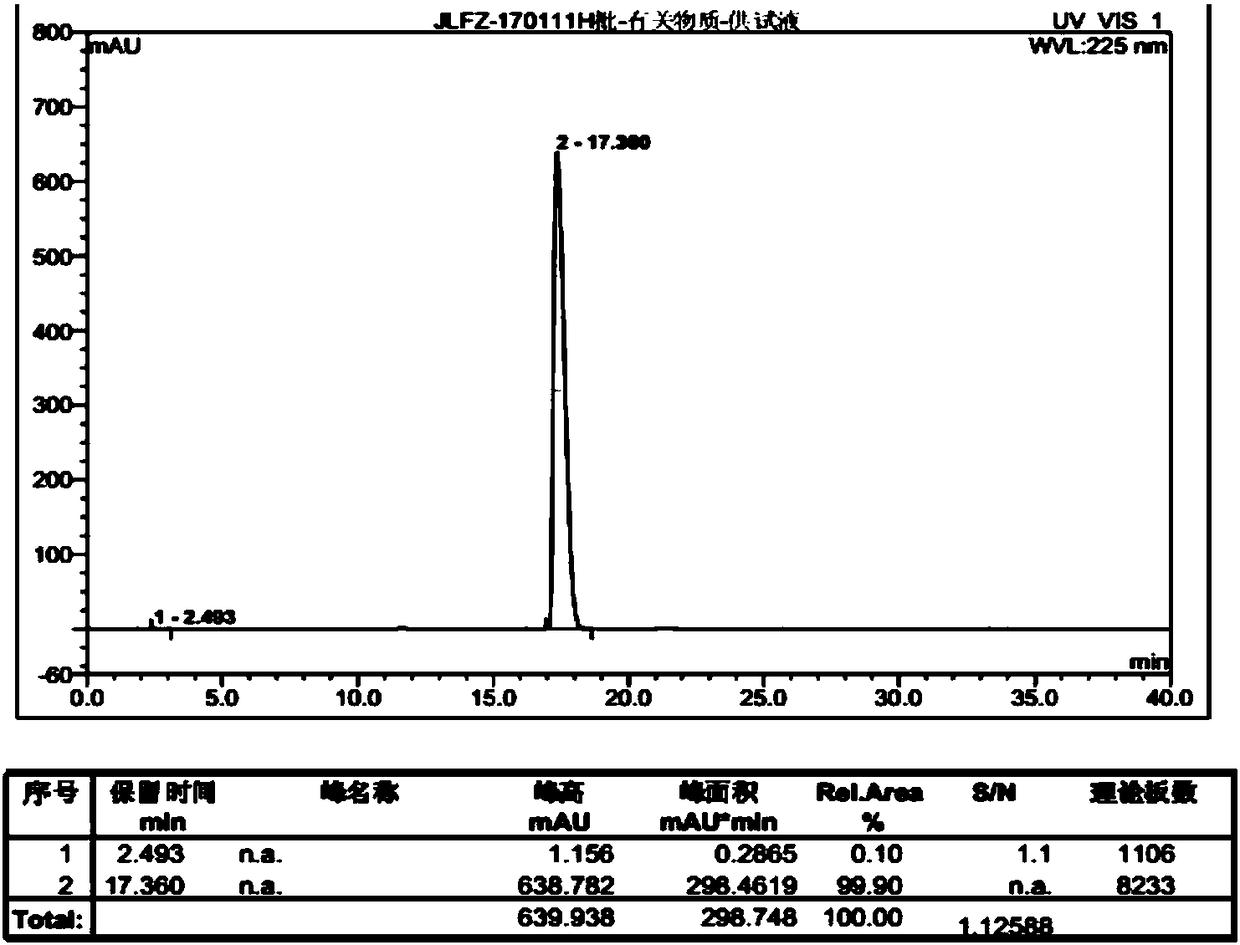
[0042] Put 50 g (0.26 mol) of p-chlorophenoxyacetic acid into dichloromethane in a single-necked bottle, and then add 47.5 g (0.40 mol) of thionyl chloride dropwise. After the dropwise addition, reflux and stir the reaction. The reaction solution was spin-dried to obtain an oil. Dichloromethane was added to the obtained oily substance, and at the same time, 26 g (0.28 mol) of dimethylaminoethanol (0.28 mol) of dichloromethane solution was added dropwise into the reaction flask, and the dropwise addition was completed in 30 minutes, and the reaction was stirred at room temperature. Water was added for extraction, and the obtained organic layer was spin-dried. The obtained oil was added to 250ml of ethyl acetate, and hydrogen chloride gas was used to form hydrochloride, until a large amount of solids were precipitated, and filtered at 25 ° C to obtain 68 g of white solids, with a yield of 87.1% and a purity of 99.9% (attached figure 2 ):
Embodiment 3~ Embodiment 10
[0044] Except that the process parameters of embodiment 3~embodiment 10 are listed in table 1, all the other steps and methods are the same as embodiment 1.
[0045] Table 1 shows the process parameters, product purity and yield of Examples 3 to 12.
[0046]
[0047] As can be seen from Table 1, the process parameters adopted in Examples 3, 4, 5, and 6 fall within the scope of the present invention, that is, the mol ratio of p-chlorophenoxyacetic acid, thionyl chloride, and dimethylethanolamine is 1 : 1.5~3.0: 1.1~1.5 These examples have all obtained good yields, and the purity is also above 99.5%.
[0048] However, Examples 7, 8, 9, and 10 did not adopt the process parameters of the present invention, and their yields and purity were obviously not as good as those of Examples 3, 4, 5, and 6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com