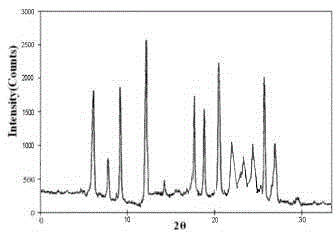
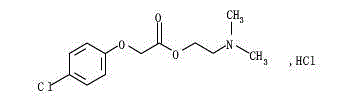
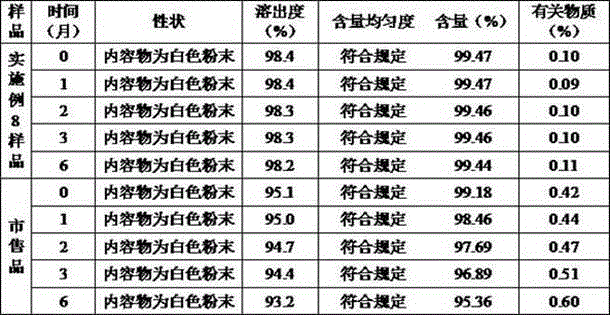
Meclofenoxate hydrochloride compound and pharmaceutical composition thereof
A technology for meclofen axetil and a compound, applied in the field of medicine, can solve the problems of high storage conditions, unstable raw material stages of easy hydrolysis of meclofen axetil, easy hydrolysis of meclofen axetil, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1 The preparation of meclofenoxate hydrochloride compound
[0048] 1. Add the raw material of meclofenoxate hydrochloride into the mixed solution of chloroform and ethanol with a volume ratio of 9:1 at a weight ratio of 1:15, adjust the temperature of the solution to 30°C, and stir to dissolve.
[0049] 2. Keep the solution temperature at 30°C, add activated carbon with a weight of 0.7% of the weight of meclofenoxate hydrochloride, stir at 120 rpm for 25 minutes, filter to remove carbon, and filter the filtrate through a 0.22 μm filter membrane to obtain the filtrate;
[0050] 3. Adjust the temperature of the filtrate to 22°C, and at a stirring speed of 90 rpm, slowly and uniformly add the weight of the mixture of chloroform and ethanol in step 1 to the filtrate obtained in step 1 at a speed of 50ml / min while stirring. Double the volume ratio of the mixed solution of diethyl ether and petroleum ether with a volume ratio of 8:2; at the same time, cool down to 8°...
Embodiment 2
[0053] Embodiment 2 Preparation of Meclofenoxate Hydrochloride Compound
[0054] 1. Add the raw material of meclofenoxate hydrochloride into the mixed solution of chloroform and ethanol with a volume ratio of 9:1 at a weight ratio of 1:18, adjust the temperature of the solution to 35°C, and stir to dissolve.
[0055] 2. Keep the solution temperature at 35°C, add activated carbon with a weight of 0.7% of the weight of meclofenoxate hydrochloride, stir at 150 rpm for 25 minutes, filter to remove carbon, and filter the filtrate through a 0.22 μm filter membrane to obtain the filtrate;
[0056] 3. Adjust the temperature of the filtrate to 27°C, and at a stirring speed of 110 rpm, slowly and uniformly drop the weight of the mixture of chloroform and ethanol in step 1 to the filtrate obtained in step 2 at a speed of 75ml / min while stirring. Double the volume ratio of the mixed solution of diethyl ether and petroleum ether with a volume ratio of 8:2; at the same time, cool down to 10...
Embodiment 3
[0059] Example 3 Preparation of Meclofenoxate Hydrochloride Freeze-dried Powder (Specification: 0.06g)
[0060] prescription:
[0061] Meclofenoxate hydrochloride compound: 60g
[0062] Mannitol: 240g
[0063] Add water for injection to 2000ml
[0064]
[0065] Made 1000 pieces
[0066] Process:
[0067] 1. Add the prescribed amount of meclofenoxate hydrochloride into 1400ml of water for injection, stir to dissolve, add water for injection to 1600ml, then add mannitol according to the prescribed amount, stir to dissolve, add water for injection to 2000ml, and stir evenly.
[0068] 2. Add 0.10% (g / ml) activated carbon to 1, stir for 20 minutes, decarbonize by filtration, sterilize by filtration with 0.22μm filter membrane, and carry out intermediate detection.
[0069] 3. Freeze-drying:
[0070] ① Pre-freezing: Put the filtrate in 2 into a half-pack and put it in a freezer that has been cooled to -24°C in advance, keep it for 1.5 hours, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com