Taxane prodrug, preparation method and application thereof
A taxane and drug technology, applied in the field of taxane drug precursors and their preparation, can solve the problems of poor drug stability, poor water solubility, low maximum tolerated dose and the like, and achieve the effect of increasing the application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] The synthesis of embodiment 1 taxane prodrug 1
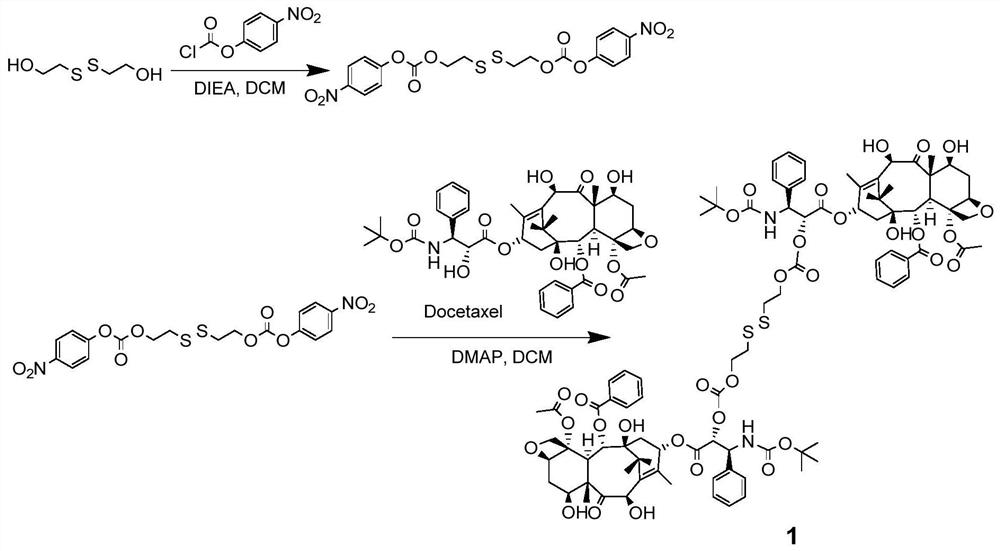
[0085] Such as figure 1 As shown, add bis(2-hydroxyethyl) disulfide (1.08g, 7.0mmol) and p-nitrophenyl chloroformate (3.10g, 15.4mmol) in a 100mL round bottom flask, and use anhydrous di Methane chloride (DCM, 8 mL) was dissolved, and N,N-diisopropylethylamine (DIEA) (2.26 g, 17.5 mmol) was added. The reaction mixture was stirred at 25°C for 5 hours, then washed with 5% aqueous citric acid, saturated aqueous sodium bicarbonate, and saturated aqueous sodium chloride. The organic layer was dried over anhydrous sodium sulfate, filtered, the filtrate was collected and the solvent was removed under reduced pressure. The solid was separated and purified by column chromatography (hexane / ethyl acetate=4:1) to obtain an intermediate product (1.64 g, 48.4%).
[0086] intermediate product 1 The H NMR nuclear magnetic data is as follows:
[0087] 1 H NMR (400MHz; Chloroform-d): δ8.28(d, J=9.2Hz, 4H), 7.39(d, J=9.2Hz, 4H), 4.58(...
Embodiment 2
[0091] The synthesis of embodiment 2 taxane prodrug 2
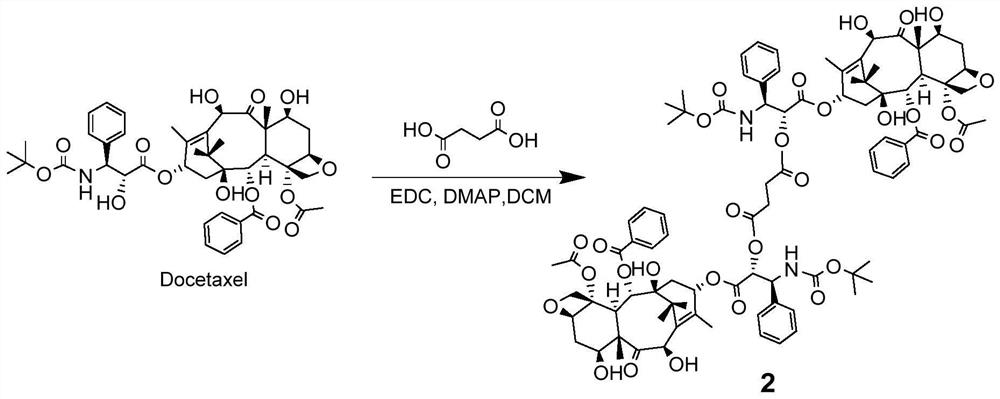
[0092] Such as figure 2 As shown, add succinic acid (7.1mg, 0.06mmol), DTX (100mg, 0.12mmol) in a 100mL round bottom flask, add 4mL of anhydrous DCM (dichloromethane) to dissolve, then add EDC (118mg, 0.76mmol ) and DMAP (4-dimethylaminopyridine) (8 mg, 0.07 mmol). Stir and react at 25°C for 24 hours, then wash with 5% citric acid, saturated sodium bicarbonate, and saturated brine respectively; dry the organic phase with anhydrous sodium sulfate, filter, collect the filtrate and remove the solvent under reduced pressure; Product 2 (309 mg, yield 65.3%) was obtained after separation and purification (n-hexane:ethyl acetate=1:1).
Embodiment 3
[0093] The synthesis of embodiment 3 taxane prodrug 3
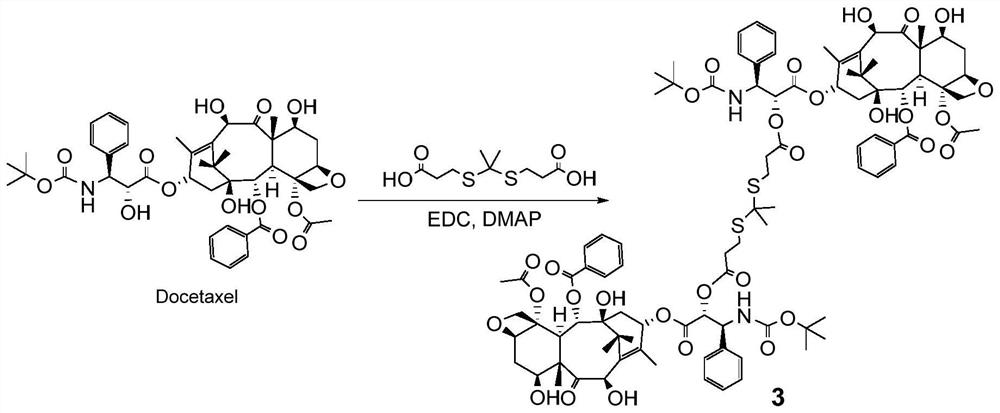
[0094] Such as image 3 As shown, add such as image 3 The indicated thioketal (50mg, 0.2mmol), DTX (355.5mg, 0.44mmol), after adding 3mL of anhydrous DCM (dichloromethane) to dissolve, then adding EDC (22.9mg, 0.14mmol) and DMAP (4- Dimethylaminopyridine) (12 mg, 0.1 mmol). Stir overnight at 45°C, then wash with 5% citric acid, saturated sodium bicarbonate, and saturated brine respectively; dry the organic phase with anhydrous sodium sulfate, filter, collect the filtrate and remove the solvent under reduced pressure; the solid is separated and purified by column chromatography (dichloromethane:methanol=1:1) to obtain product 3 (128.5 mg, yield 58.5%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com