Application of DIM and derivative thereof in preparing drug for preventing and controlling injuries caused by chemotherapy
A technology of chemotherapeutic drugs and derivatives, which is applied in the field of biomedicine, can solve the problems such as no auxiliary drugs for treatment and chemotherapy, and achieve the effect of inhibiting inflammation, stable properties, and easy access
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
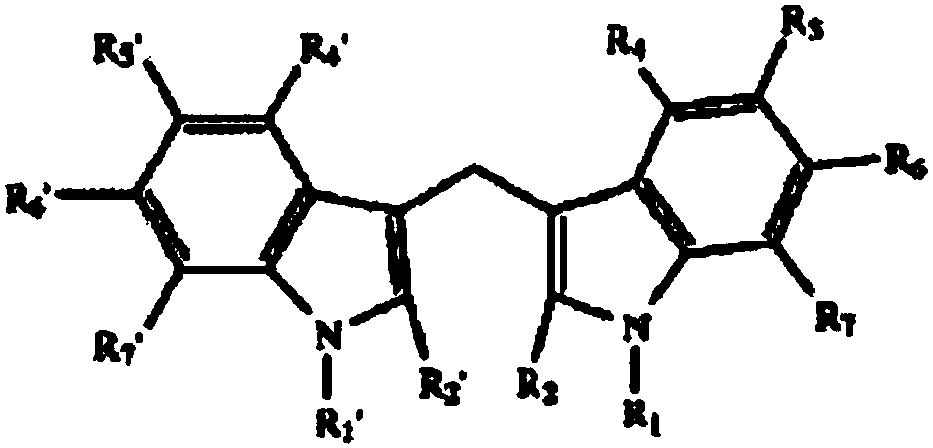
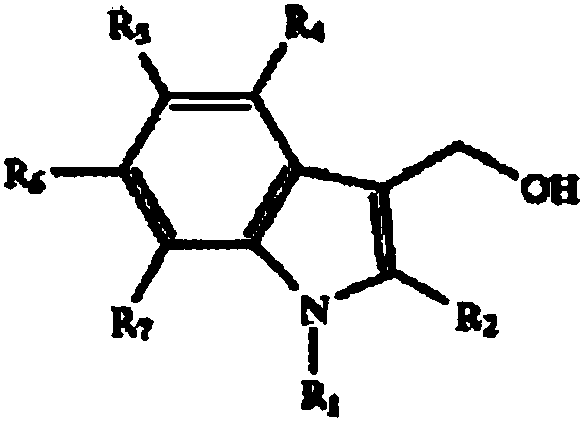
[0049] 3, the preparation of 3'-diindolylmethane and indole-3-carbinol and derivatives thereof, the specific preparation steps are as follows:
[0050] 1) Indole derivatives (eg, 5-methoxy, 5-chloro, 5-bromo, 5-fluoro, 5'-methyl, 5-nitro, N-methyl and 2-methylindole ) can be purchased commercially (Nanjing Ruima Fine Chemical Co., Ltd.), and the substituted indole-3-acetaldehyde product is derived by reducing the aldehyde group with a suitable alcohol such as methanol and sodium borohydride to give I3C. 2.9ml of dimethylformamide was cooled to 0°C in an ice-salt bath, then slowly added 0.86ml of phosphorus oxychloride (more than 30min), and 8.6mmol of indole derivatives were dissolved in 1.0ml of dimethylformamide , and then slowly add it to the previously cooled phosphorus oxychloride solution (time greater than 10min), the formed suspension is heated at 37°C for 60min-90min until the clear yellow solution becomes a slightly yellowish paste, and then to Add 1ml of ice water ...
Embodiment 2
[0054] Application of oral 3,3'-diindolylmethane, indole-3-carbinol and derivatives thereof in the preparation of prevention and treatment of liver, kidney and digestive tract tissue damage caused by chemotherapy drugs.
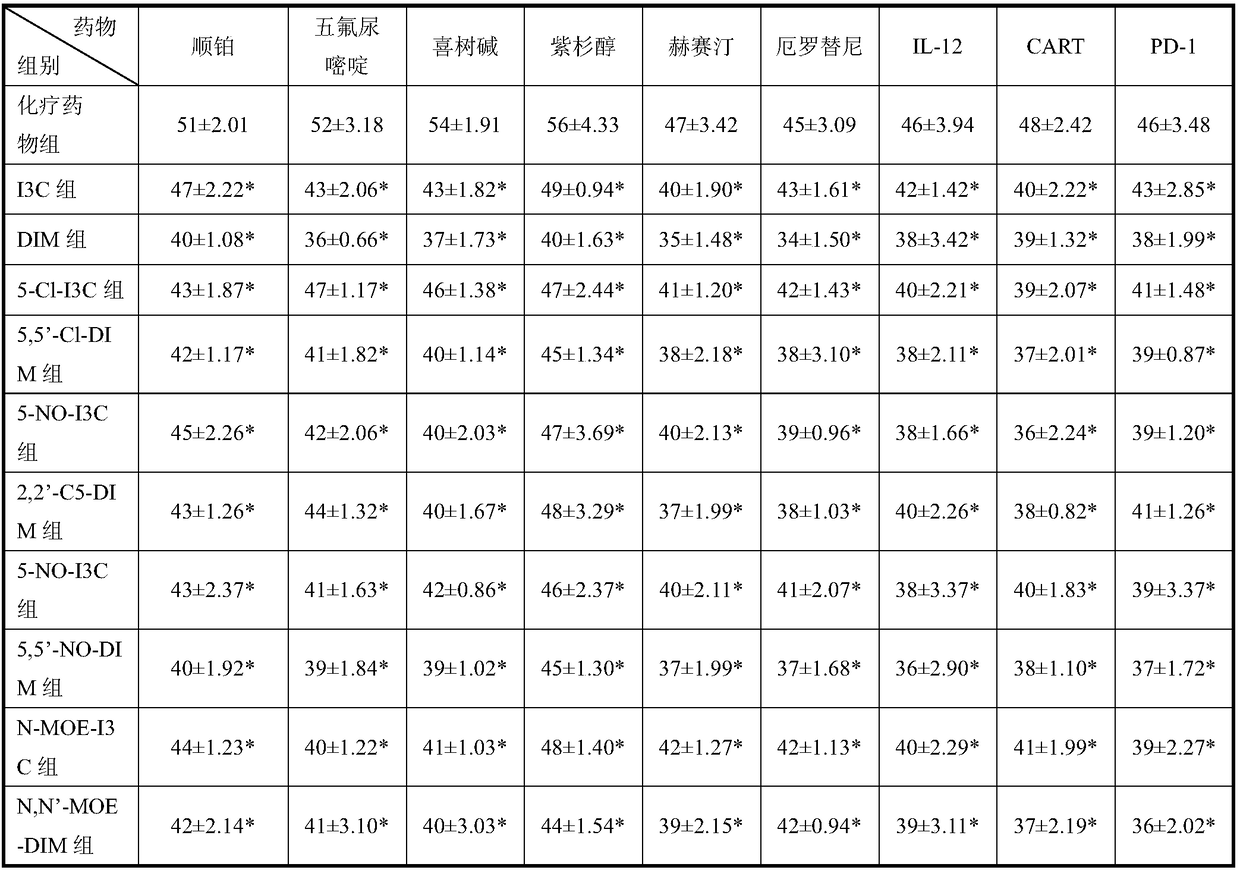
[0055] I3C, DIM, and its derivative compounds 5-chloroindole-3-methanol (5-Cl-I3C), 5,5'-chlorodiindolylmethane (5,5'-Cl-DIM), 2-pentane Indole-3-carbinol (2-C5-I3C), 2,2'-pentyl-diindolylmethane (2,2'-C5-DIM), 5-nitroindole-3-carbinol (5 -NO-I3C), 5,5'-nitrodiindolylmethane (5,5'-NO-DIM), N-methoxyindole-3-methanol (N-MOE-I3C) and N,N '-Methoxy-diindolylmethane (N,N'-MOE-DIM), respectively dissolved in corn oil, prepared as a 2.0 mg / ml stock solution for future use.
[0056] 1. Experimental animals
[0057] Clean-grade male ICR mice, weighing 16-18 g, were purchased from Beijing Weitong Lihua Company and raised in the Experimental Animal Center of Nanjing University.
[0058] 2. Experimental grouping and processing
[0059]Mice were randomly divided into...
Embodiment 3
[0097] Application of intraperitoneal injection of aqueous solution preparations of 3,3'-diindolylmethane, indole-3-carbinol and their derivatives in the preparation of prevention and treatment of liver, kidney and digestive tract tissue damage caused by chemotherapy drugs.
[0098] I3C, DIM, and its derivative compounds 5-chloroindole-3-methanol (5-Cl-I3C), 5,5'-chlorodiindolylmethane (5,5'-Cl-DIM), 2-pentane Indole-3-carbinol (2-C5-I3C), 2,2'-pentyl-diindolylmethane (2,2'-C5-DIM), 5-nitroindole-3-carbinol (5 -NO-I3C), 5,5'-nitrodiindolylmethane (5,5'-NO-DIM), N-methoxyindole-3-methanol (N-MOE-I3C) and N,N '-Methoxy-diindolylmethane (N,N'-MOE-DIM), prepared with cyclodextrin and physiological saline to prepare a stock solution of 1.0 mg / kg for future use.
[0099] 1. Experimental animals
[0100] Clean-grade male ICR mice, weighing 16-18 g, were purchased from Beijing Weitong Lihua Company and raised in the Experimental Animal Center of Nanjing University.
[0101] 2. Expe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com