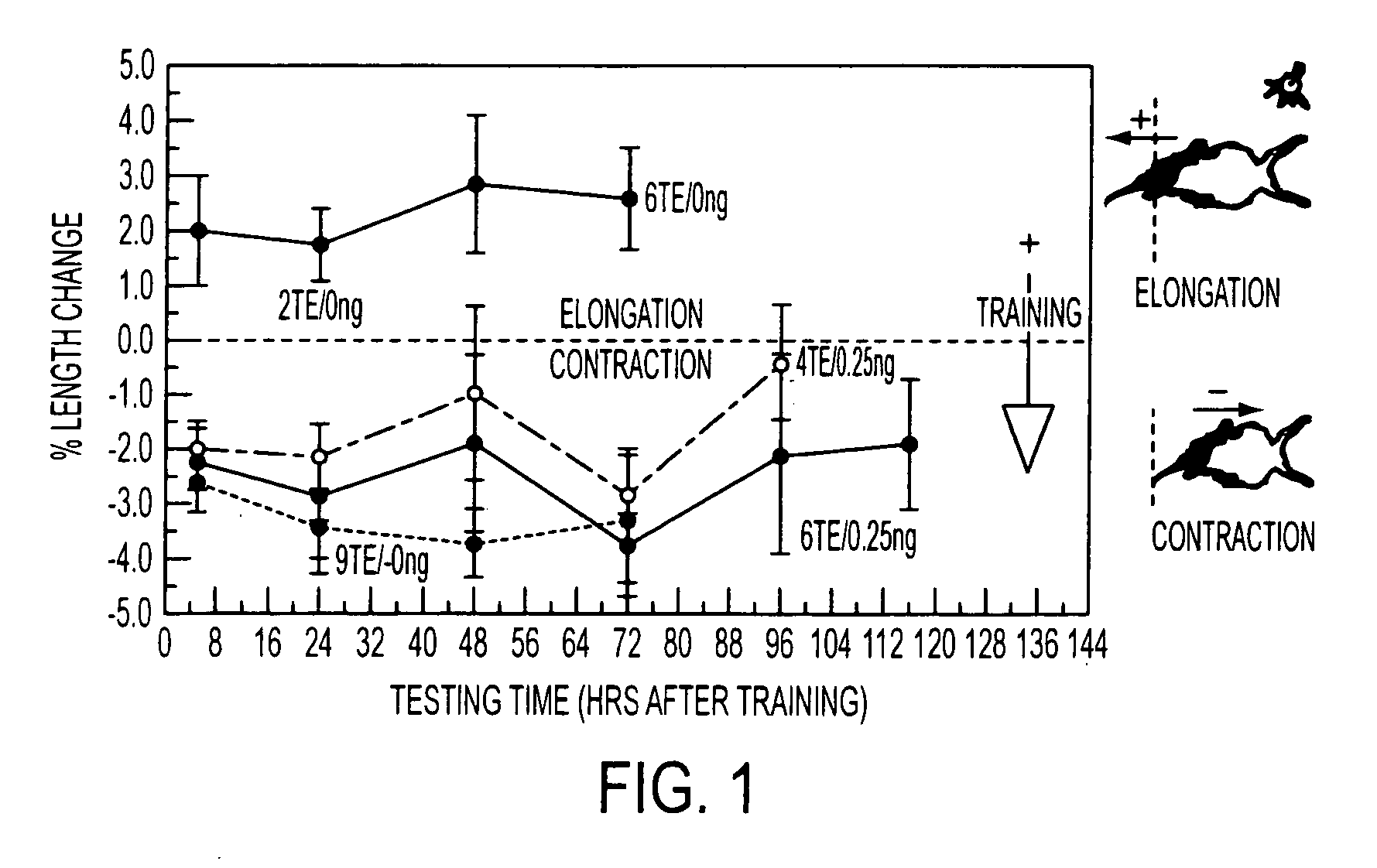
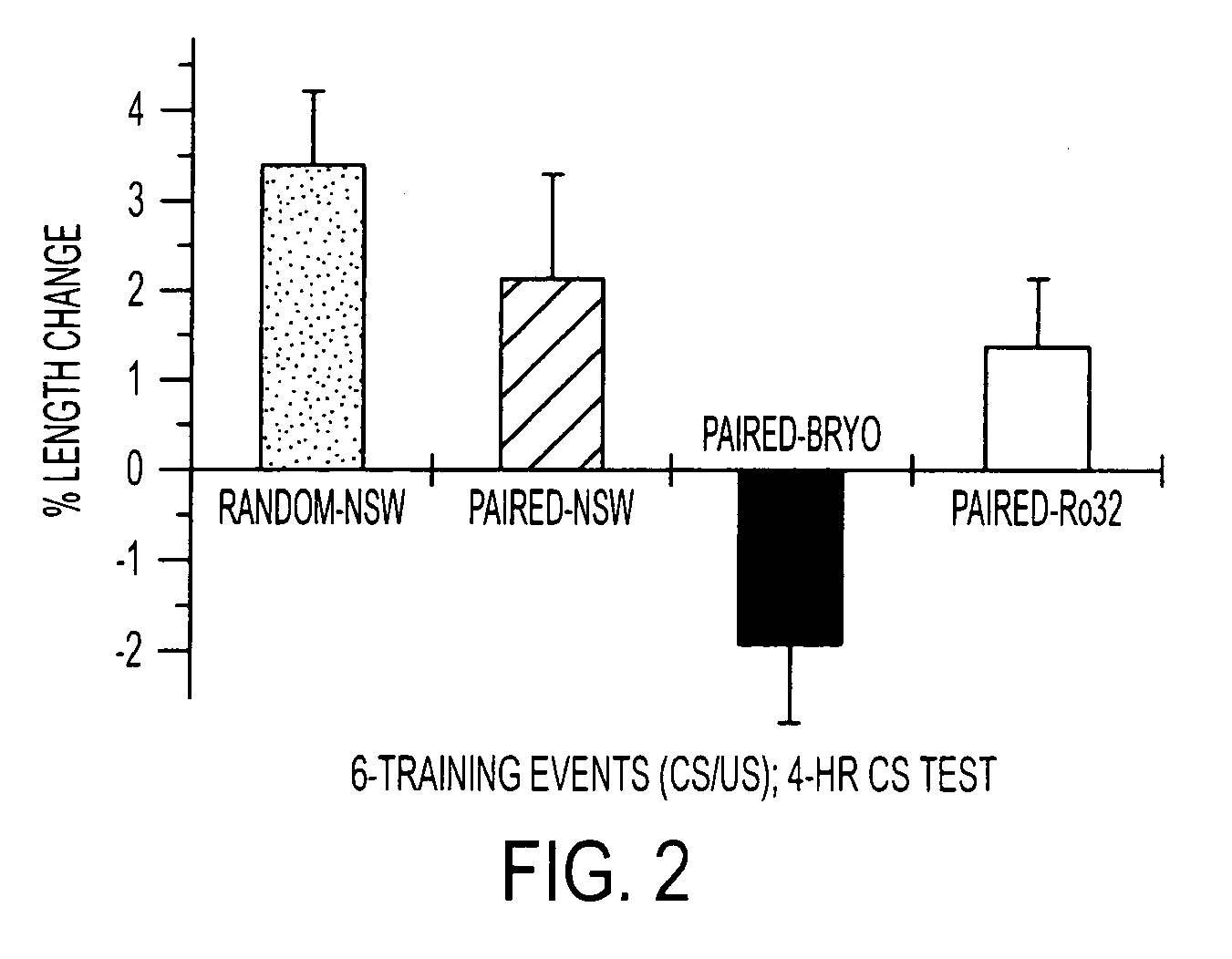
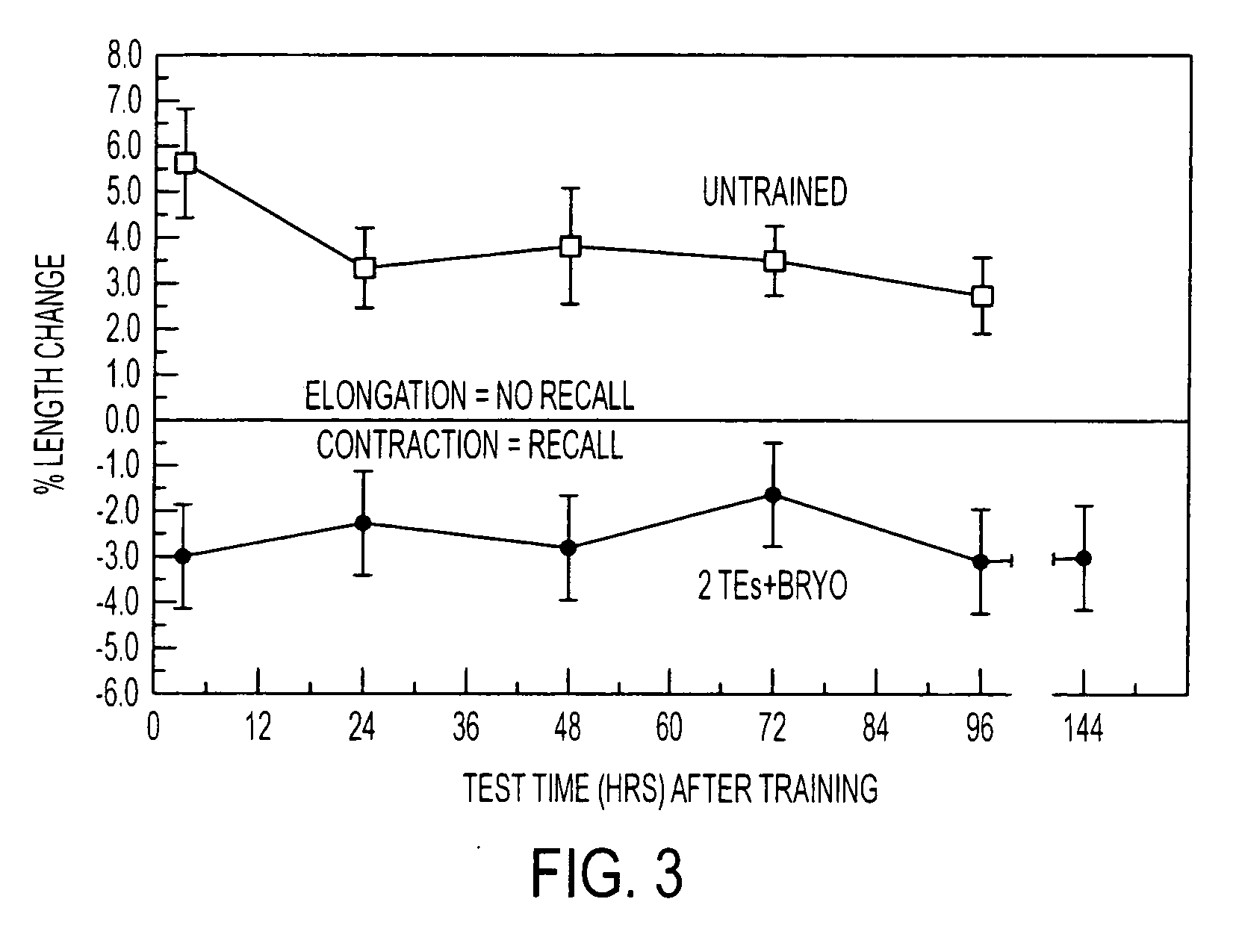
Protein synthesis required for long-term memory is induced by PKC activation on days preceding associative learning
a protein synthesis and associative learning technology, applied in the direction of biocide, heterocyclic compound active ingredients, drug compositions, etc., can solve the problems of long-term antagonism and complicated approach, and achieve the effect of reducing myalgia, reducing myalgia produced, and preferentially inhibiting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Behavioral Pharmacology
[0076] Bryostatin exposure—Specimens of Hermissenda Crassicornis were maintained in artificial sea water (ASW) at 15° for three days in perforated 50-ml conical centrifuge tubes before starting experiments. Bryostatin, purified from the marine bryozoan Bugula neritina, was dissolved in EtOH and diluted to its final concentration in ASW. Animals were incubated with bryostatin in ASW for 4 hr, then rinsed with normal ASW. For selected experiments lactacysteine (10 μM) or anisomycin was added to the ASW.
[0077] Bryostatin effects on Hermissenda behavior and biochemistry were produced by adding the drug to the bathing medium within an 8 cm long, 1 cm diameter test tube housing each individual animal.
example 2
Immunostaining Methods
[0078] Following experimental treatments and testing, animals were rapidly decapitated, the central nervous systems (CNS) removed and then fixed in 4% para-formaldehyde in 20 mM Tris-buffered (pH 8) natural seawater (NSW; 0.2 μm micropore-filtered). The CNSs were then embedded in polyester wax (20), sectioned (6 μm) and immunostained using a biotinylated secondary antibody coupled to avidin-bound microperoxidase (ABC method, Vector), Aminoethylcarbazole (AEC) was used as the chromogen. The primary polyclonal antibody (designated 25U2) was raised in rabbits from the full length calexcitin protein extracted from squid optic lobes. Gray-scale intensity measures were done from digital photomicrographs on circumscribed cytoplasmic areas of the B-photoreceptors minus the same background area (non-staining neuropile).
example 3
[0079] Cells were homogenized by sonication (5 sec, 25 W) in 100 μl of 10 mM Tris-HCl pH 7.4 buffer containing 1 mM EGTA, 1 mM PMSF, and 50 mM NaF. Homogenate was transferred to a polyallomer centrifuge tube and was centrifuged at 100,000×g for 10 min at 4°. The supernatant was removed and immediately frozen on dry ice. The particulate fraction was resuspended by sonication in 100 μl of the same buffer and stored at −80°. To measure PKC, 10 μl of cytosol or particulate fraction was incubated for 15 min at 37° in the presence of 10 μM histones, 4.89 mM CaCl2, 1.2 μg / μl phosphatidyl-L-serine, 0.18 μg / μl 1.2-dioctanoyl-sn-glycerol, 10 mM MgCl2, 20 mM HEPES (pH 7.4), 0-8 mM EDTA, 4 mM EGTA, 4% glycerol, 8 μg / ml aprotinin, 8 μg / ml leupeptin, and 2 mM benzamidine. 0.5 μCi [γ32P]ATP was added and 32P-phosphoprotein formation was measured by adsorption onto phosphocellulose as described previously (25). This assay was used with slight adjustments for either Hermissend...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com