Method for preparing matrine slow-release tablet by applying attapulgite
A technology of attapulgite and modified attapulgite, which is applied to medical preparations containing active ingredients, pharmaceutical formulas, allergic diseases, etc., and can solve problems such as increased side effects, high cost, and complicated excipients, and achieve increased added value , Low production cost and short production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
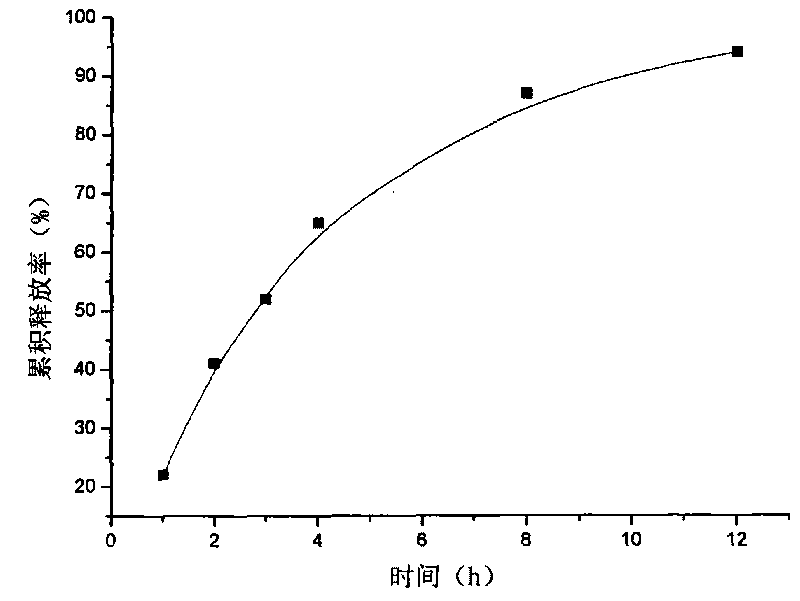
[0017] Example 1: Weigh 20 g of attapulgite and place it in 1000 ml of aqueous solution, stir for 12 hours, let stand, pour off the upper layer of water, add purified water, stir for 12 hours, let stand, take the middle fine particle layer, add concentrated hydrochloric acid and let stand for 24 hours, Suction filtration, washing to neutrality, drying at 105°C, grinding, passing through a 120 mesh sieve to obtain acid-modified attapulgite; take 20 g of matrine, and dissolve it into a 2 mg / ml solution with hydrochloric acid with a mass concentration of 0.1%. Matrine solution was absorbed by 40g of acid-modified attapulgite for 4h, filtered, and vacuum-dried at 60°C to obtain attapulgite loaded with matrine; add 2% of its weight talcum powder to the attapulgite loaded with matrine Mix evenly, dry granulate, sieve and granulate, and tablet, making 100 tablets in total.
Embodiment 2
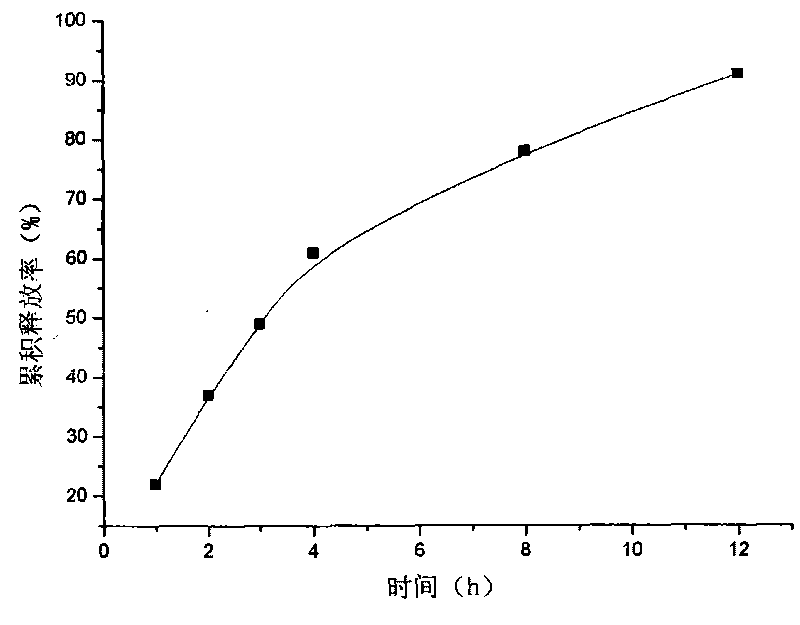
[0018] Example 2: Weigh 20 g of attapulgite and place it in 1000 ml of aqueous solution, stir for 12 hours, let stand, pour off the upper layer of water, add purified water, stir for 12 hours, let stand, take the middle fine particle layer, add concentrated hydrochloric acid and let stand for 24 hours, Suction filtration, washing to neutrality, drying at 105°C, fine grinding, and passing through a 120 mesh sieve to obtain acid-modified attapulgite; take 40 g of matrine, and dissolve it into a 3 mg / ml solution with hydrochloric acid with a mass concentration of 0.1%. The matrine solution was absorbed by 80 g of acid-modified attapulgite for 5 hours, filtered, and vacuum-dried at 60° C. to obtain attapulgite loaded with matrine; 2% of its weight of talcum powder was added to the attapulgite loaded with matrine Mix evenly, dry granulate, sieve and granulate, compress into tablets, and make 200 tablets in total.
Embodiment 3
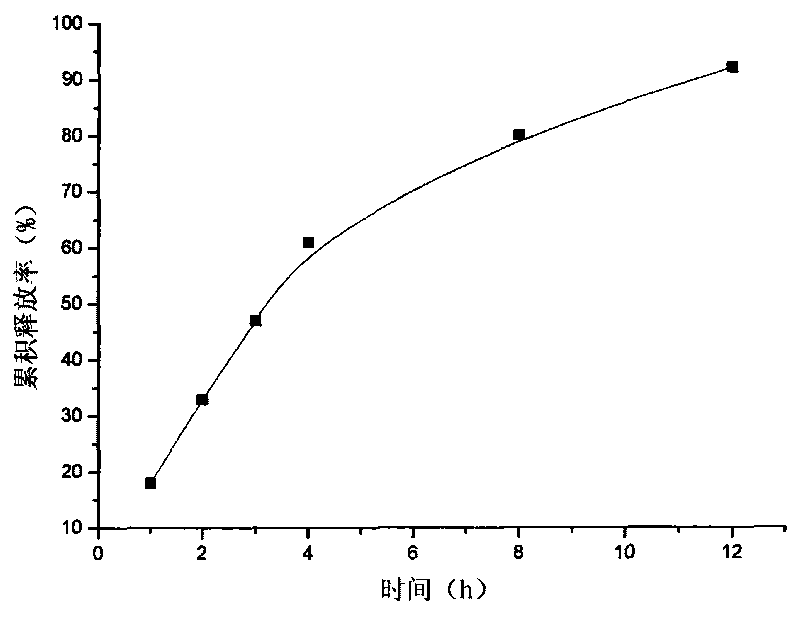
[0019] Example 3: Take attapulgite 20g and place it in 1000ml aqueous solution, stir for 12h, let it stand, pour off the upper layer of water, add pure water, stir for 12h, let stand, take the middle fine particle layer, add concentrated hydrochloric acid and let stand for 24h, Suction filtration, washing to neutrality, drying at 105°C, grinding finely, and passing through a 120 mesh sieve to obtain acid-modified attapulgite; take 40 g of matrine, and dissolve it into a 5 mg / ml solution with hydrochloric acid with a mass concentration of 0.1%. The matrine solution was absorbed by 80 g of acid-modified attapulgite for 6 hours, filtered, and vacuum-dried at 60°C to obtain matrine-loaded attapulgite; add 2% of its weight to the acid-modified attapulgite loaded with matrine The talc powder was mixed evenly, dry granulated, sieved and granulated, and compressed into tablets to make 200 tablets in total.
[0020] Content determination: Take a piece of sustained-release tablet, grind...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com