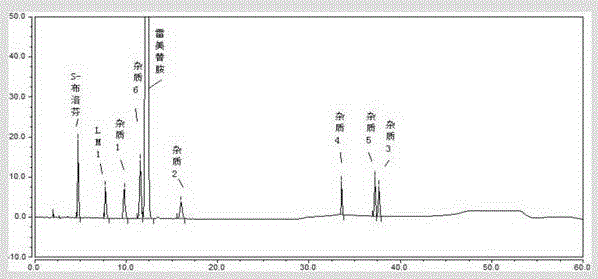
Patents
Literature
45results about How to "Ensure quality controllability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for industrially preparing dimorphic hard alloy with both high strength and high tenacity
The invention discloses a method for industrially preparing a dimorphic hard alloy with both high strength and high tenacity, belonging to the technical field of hard alloys. The method comprises the following steps of: according to the content of Co in the dimorphic hard alloy, ball-milling and mixing WO2.9, Co3O4 and carbon black; coldly pressing into a billet block; preparing WC-Co composite powder in a vacuum furnace; ball-milling by taking absolute ethyl alcohol or hexane as a medium; drying to obtain the WC-Co composite powder; under the protection of argon, performing powder aggregation pre-treatment; raising the temperature to 650-950 DEG C at a speed of 5-8 DEG C per minute; keeping the temperature for 30-60 min; adding 30-80 ml of polyethylene glycol forming agent to each kilogram of powder; moulding and forming; and sintering the moulded and formed powder billet in vacuum or at low pressure. The WC-Co dimorphic hard alloy prepared by the invention has both high strength andexcellent breakage tenacity. The method is an integral industrialization preparation technology route.
Owner:BEIJING UNIV OF TECH
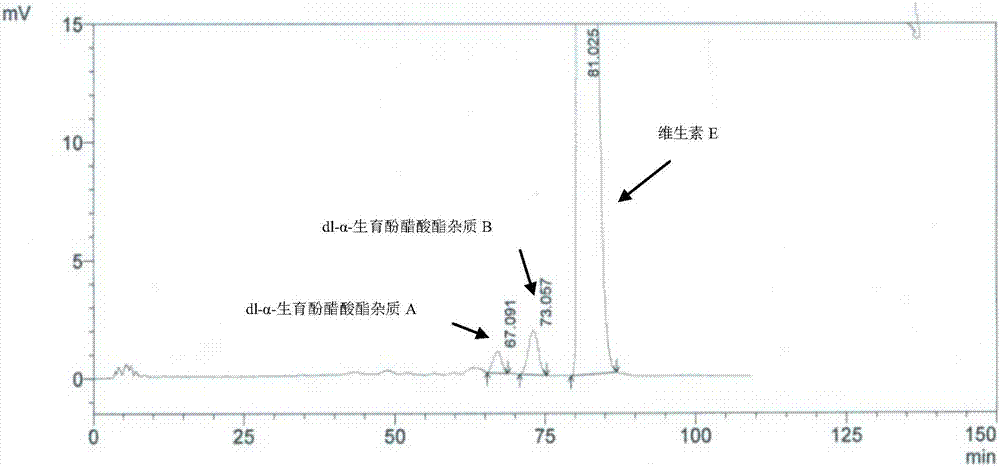
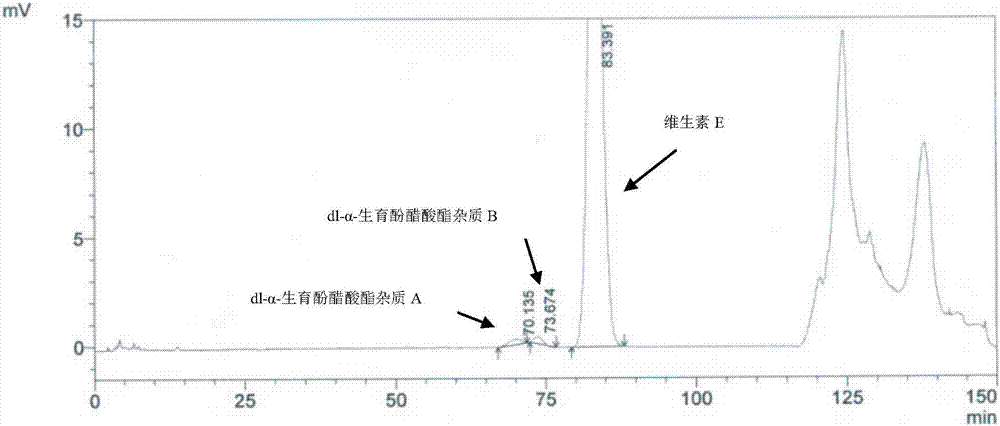
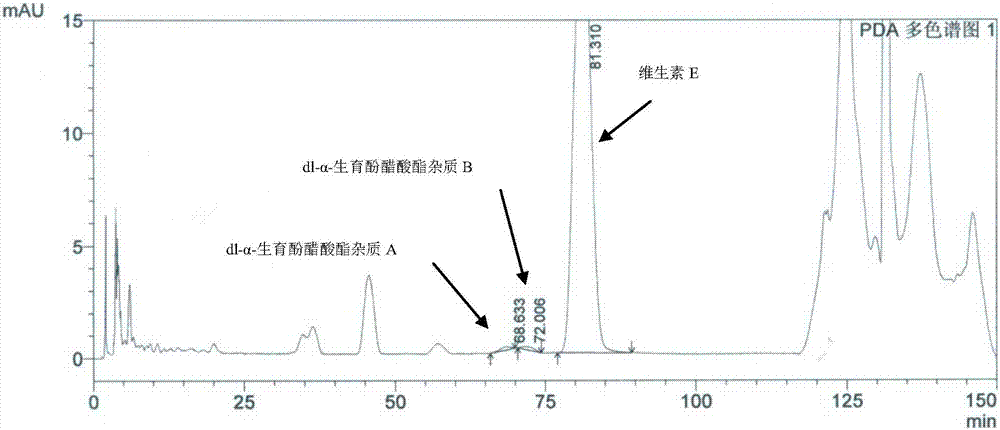
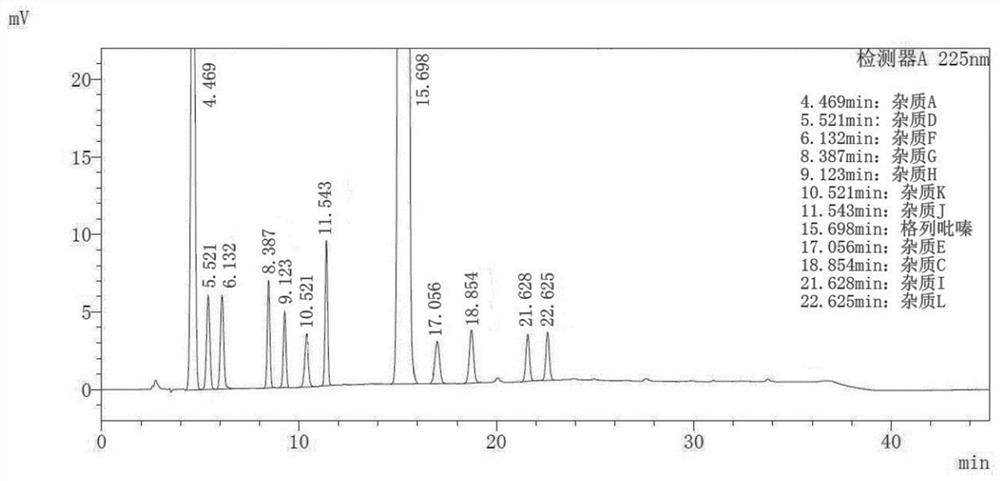
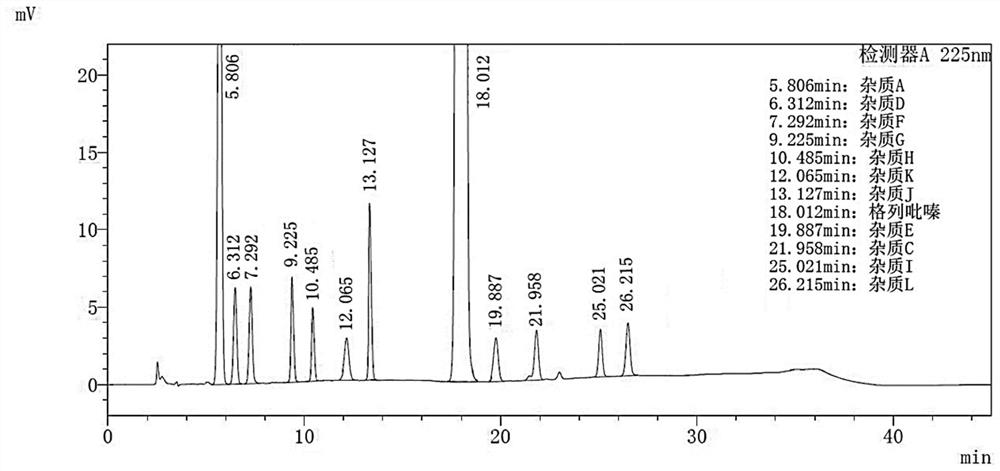
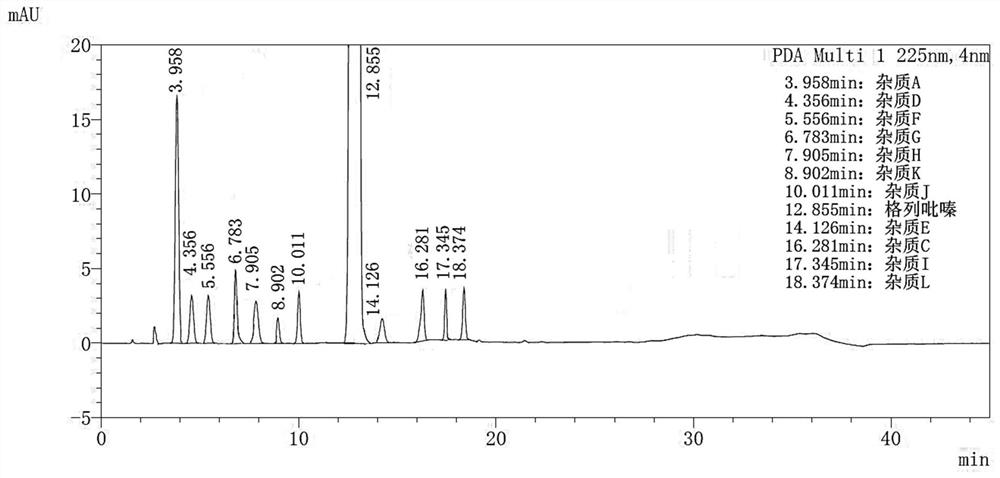
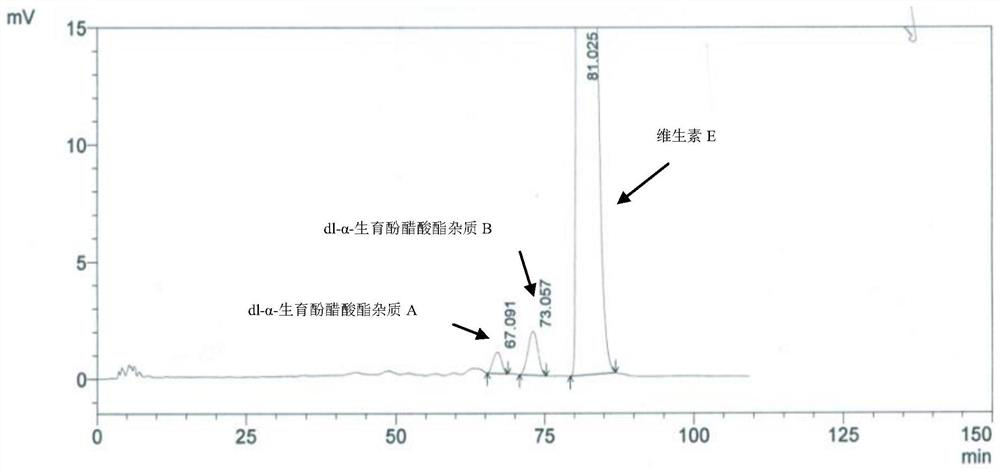
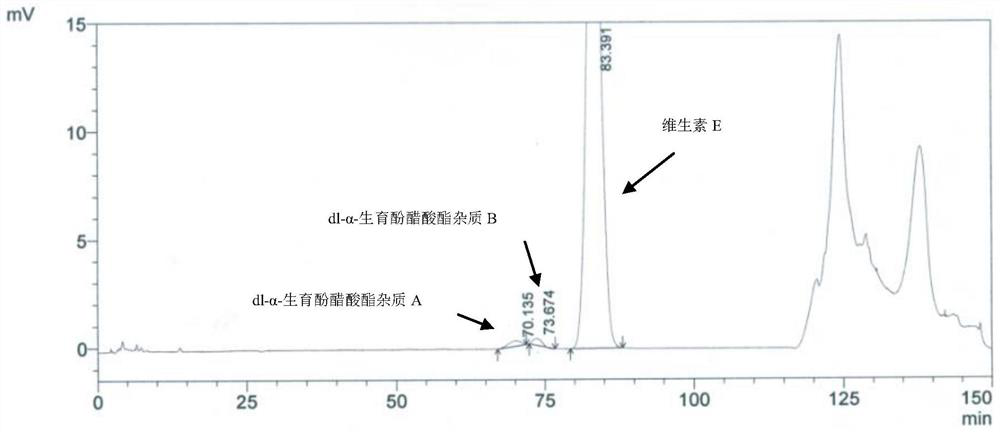
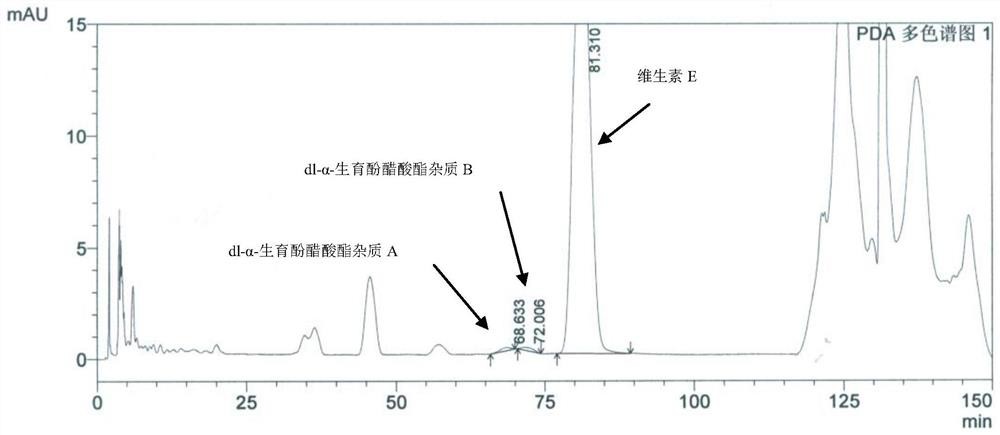
Method for separating and determining impurities in vitamin E and preparation thereof with HPLC (High Performance Liquid Chromatography) method
ActiveCN107202849AHigh detection sensitivityEnsure quality controllabilityComponent separationSolventChemistry
The invention discloses a method for separating and determining impurities in vitamin E and a preparation thereof with an HPLC (High Performance Liquid Chromatography) method. The method comprises the following steps: chromatograph condition: by taking octadecylsilane bonded silica gel as filler, a mixed solution of acetonitrile, alcohol and water as a mobile phase A and a mixed solution of acetonitrile, alcohol and methanol as a mobile phase B, performing gradient elution, wherein a detection wavelength is 260-310nm, a column temperature is 25-45 DEG C, a flow rate of each mobile phase is 0.5-2.0mL / min, and a sample feeding quantity is 20-150 microlitres; preparation of a sample solution, adopting a polar solvent to prepare a d1-alpha-tocopherol acetate peak identification control solution with the concentration capable of being 0.5-3mg / mL; and determination: pouring the solution in a high performance liquid chromatograph, and recording and analyzing a chromatogram. By utilizing the method provided by the invention, quantitative analysis of related substances of the vitamin E as a crude drug and the preparation thereof can be accurately performed, and thus the quality controllability of the vitamin E and the preparation thereof is ensured.
Owner:CHINESE MEDICINES GUANGZHOU
Method for analyzing 6-ethylchenodeoxycholic acid and synthetic intermediate thereof by high-performance liquid chromatography
ActiveCN107917972AHigh detection sensitivityGuaranteed stabilityComponent separationSilanesSilica gel
The invention belongs to the technical field of pharmaceutical analysis and relates to a method for analyzing 6-ethylchenodeoxycholic acid and a synthetic intermediate thereof by high-performance liquid chromatography. The separation and detection method provided by the invention adopts an octadecyl silane bonded silica gel packed column with the chromatographic column temperature of 35-40 DEG C and a differential detector, and a phosphate buffer solution and an acetonitrile and methanol mixed solution are used for elution. The method provided by the invention realizes simple, quick and accurate separation and detection of the 6-ethylchenodeoxycholic acid and the synthetic intermediate thereof, and solves the problem of separation and detection of 6-ethylchenodeoxycholic acid-containing raw materials and preparations, thereby ensuring the quality controllability of the 6-ethylchenodeoxycholic acid and the 6-ethylchenodeoxycholic acid-containing compositions or preparations.
Owner:JIANGSU SKYRUN PHARMA CO LTD
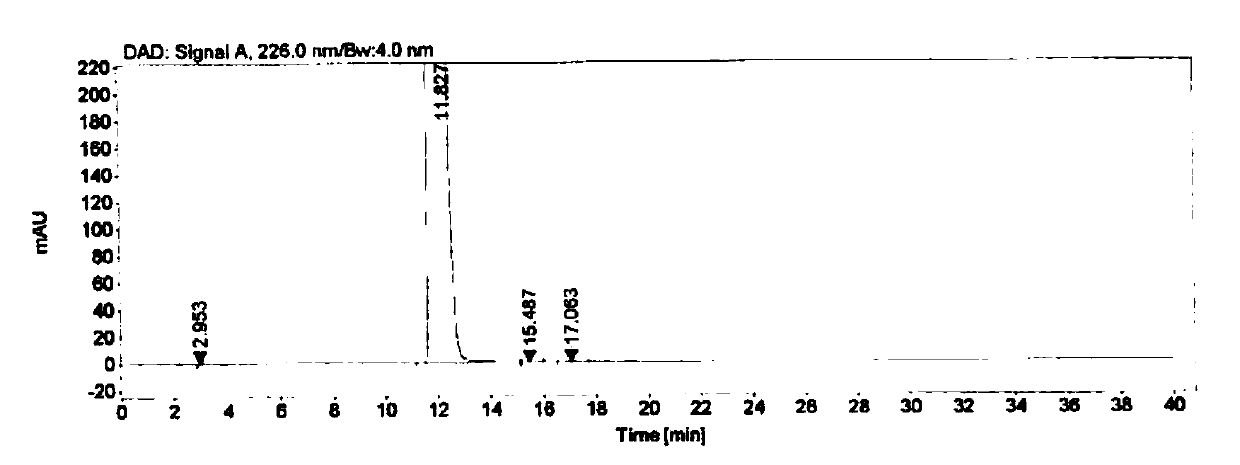
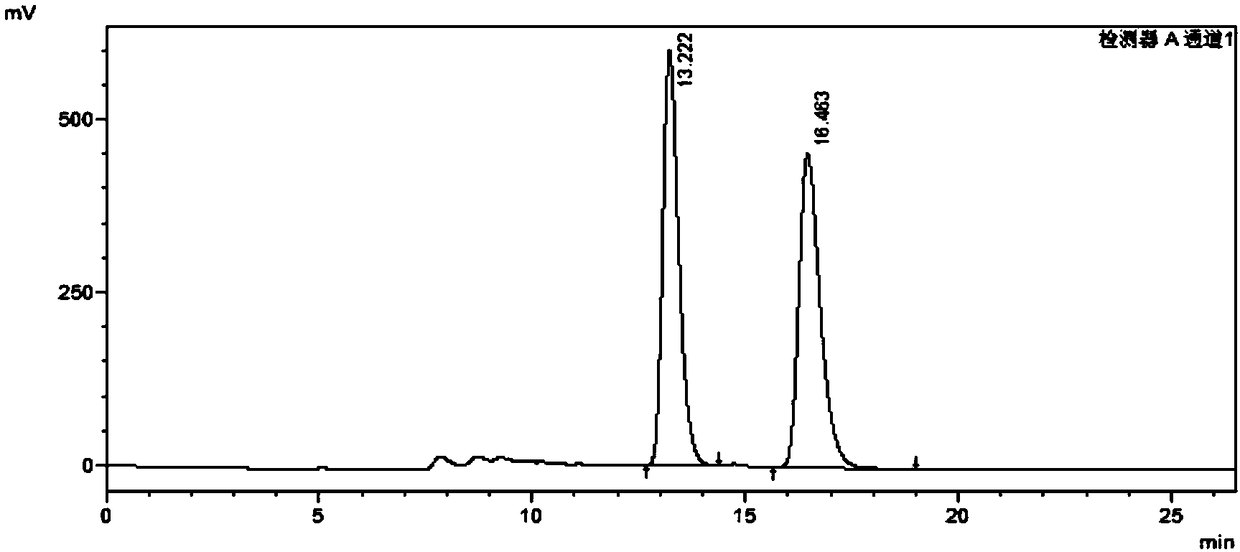
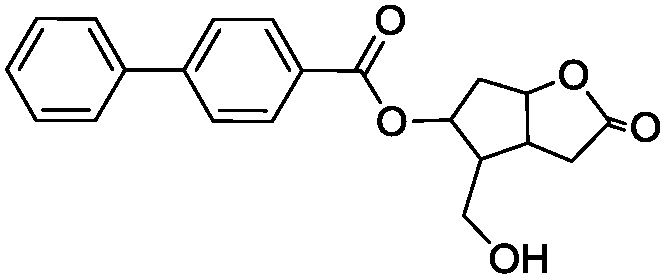
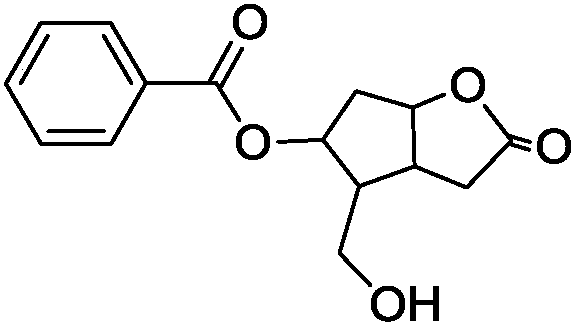
Method for analyzing and separating Corey lactone benzoate enantiomers through HPLC
The invention discloses a method for analyzing and separating Corey lactone benzoate enantiomers through an HPLC by means of a direct-chain starch type chiral chromatographic column with positive phase mixed solvent as the flowing phase. The method can easily, accurately and efficiently analyze and separate the Corey lactone benzoate enantiomers, and therefore the quality of the Corey lactone benzoate enantiomers is controlled. The method can also be used for preparing high-purity single-optical-activity Corey lactone benzoate.
Owner:吉林百纯化学科技有限公司
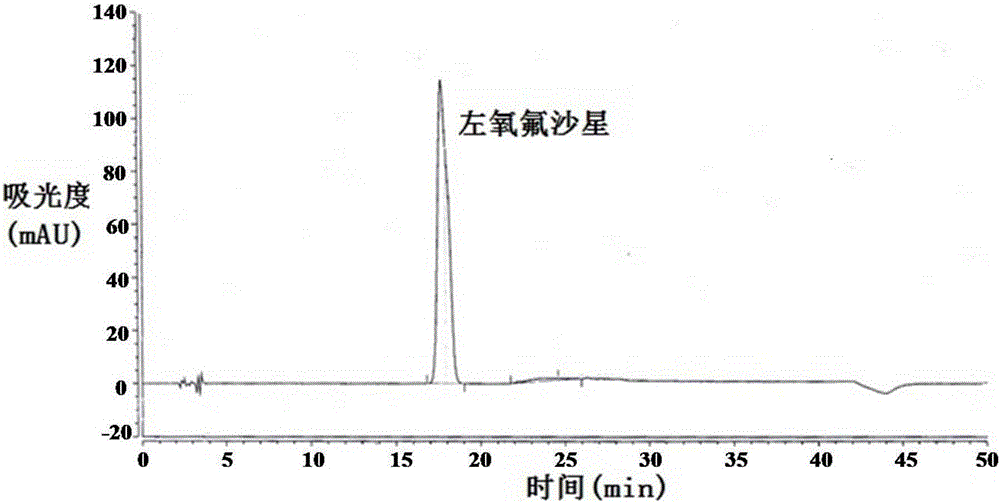
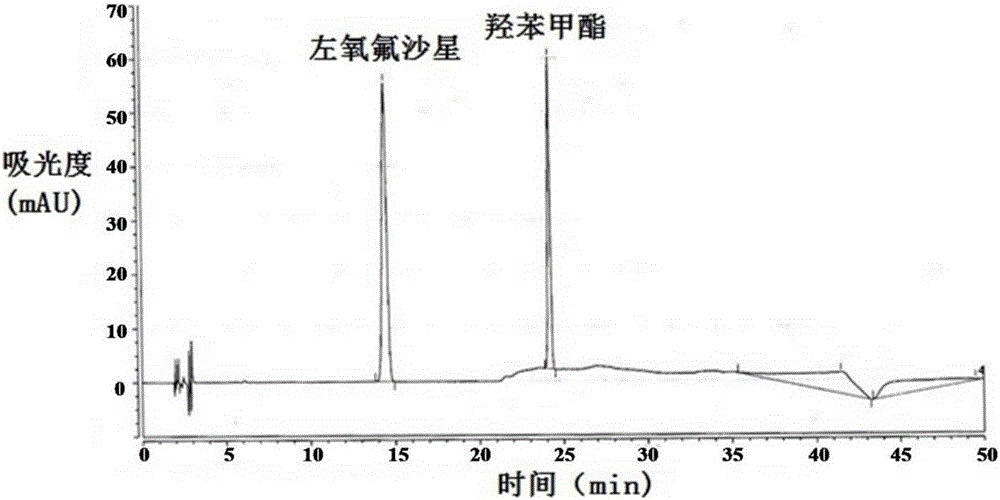
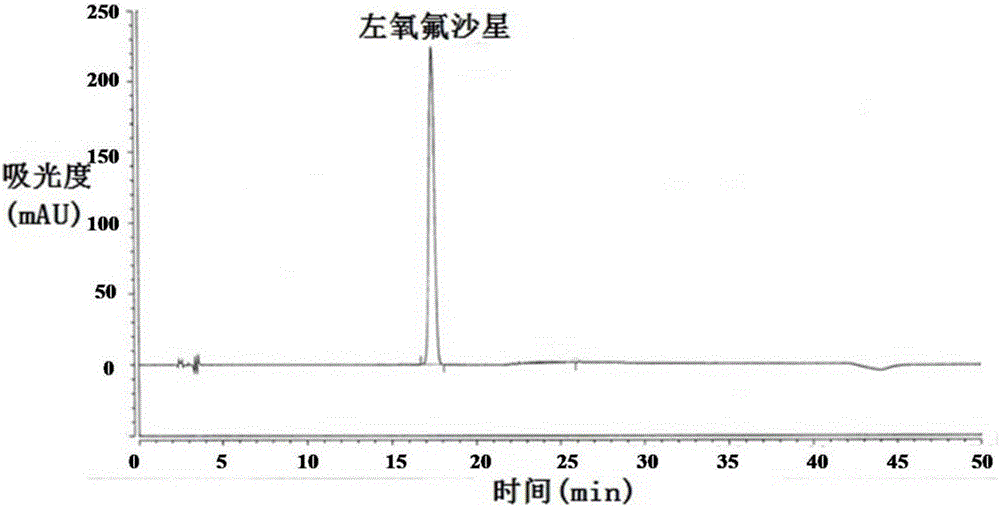
Method for separating and/or detecting levofloxacin and methylparaben in levofloxacin hydrochloride ophthalmic gel
ActiveCN106769963AEnsure quality controllabilityGuaranteed effective contentColor/spectral properties measurementsChromatographic separationSilanes
The application discloses a method for separating and / or detecting levofloxacin and methylparaben in levofloxacin hydrochloride ophthalmic gel. The method is a high performance liquid chromatography, and concretely comprises the following steps: preparing a test solution and a reference solution; using octadecyl silane bonded silica gel as a chromatographic column, wherein chromatographic separation test conditions are as follows: the column temperature is 30-40 DEG C, the flow velocity of a mobile phase is 0.9-1.3mL / min, and the ultraviolet detection wavelengths of the levofloxacin and the methylparaben are respectively 240-260nm and 280-300nm; detecting: respectively and precisely absorbing 10mu L of the test solution and 10mu L of the reference solution, injecting into a high performance liquid chromatograph, and reading the chromatographic peak areas of the levofloxacin and the methylparaben in the test solution. The method can accurately and rapidly measure the contents of the levofloxacin and the methylparaben in the levofloxacin hydrochloride ophthalmic gel at the same time, thus providing guarantee for the quality controllability of the levofloxacin hydrochloride ophthalmic gel.
Owner:湖北远大天天明制药有限公司
Quality detection method of ficuslyrata
InactiveCN109085258AGuaranteed stabilityEnsure quality controllabilityComponent separationThin layerControllability
The invention discloses a quality detection method of ficuslyrata, which comprises the steps of performing thin-layer qualitative identification on psoralen and bergamot lactone, and determining the content by using a high-performance liquid phase. The thin-layer identification method has strong specificity and no negative interference. The content detection method has a higher specificity and durability, the accuracy, reproducibility and stability of the invention can all meet the requirements of scientific research and production, and the stability and controllability of product quality canbe effectively ensured.
Owner:ZHONGSHAN TRADITIONAL CHINESE MEDICINE HOSPITAL
Traditional Chinese medicine granules for treating epigastric pain and preparation method thereof
ActiveCN102319388ANo side effectsPromote healingDigestive systemPlant ingredientsModern medicineSide effect
The invention, belonging to the field of Chinese herbal medicine, relates to traditional Chinese medicine granules for treating epigastric pain and a preparation method thereof. The granules originate from an ancestral secret recipe of a famous old herbalist doctor, and are suitable for various stomach troubles and epigastric pain caused by various reasons, especially have obvious clinic curativeeffect on stomachache, gastrectasia, anorexia, dyspepsia and other clinical symptoms. Many years clinical application proves that the curative effect is obvious. The granules have wide adaptability, substantial curative effect, and no toxic and side effect, treat both the incidental and fundamental aspects simultaneously, and have the characteristics of reasonable composition, advanced technology, convenient taking, small dosage, controllable quality, safety, reliability and the like. The granules have effects of promoting digestion, transforming stagnation, rectifying qi, harmonizing the stomach, relaxing vein, relieving pain and the like, and can be used for acute and chronic gastricism, gastroduodenal ulcer, atrophic gastritis, functional dyspepsia, prevention of stomach cancer, and the palliative treatments of related symptoms of stomach cancer patients. The granules are prepared by combining traditional Chinese medicine experiences and modern medicine production technology tightly, and are worth to be popularized and applied.
Owner:解德俊
A method for separation and determination of Raltitrexed and impurities of the Raltitrexed by high performance liquid chromatography
ActiveCN109283262AAchieve separationHigh detection sensitivityComponent separationRaltitrexedPhosphoric acid
The invention discloses a method for separation and determination of Raltitrexed and impurities of the Raltitrexed by high performance liquid chromatography. Chromatographic conditions of the method are that octadecylsilane chemically bonded silica is taken as a filler, 0.005 mol / L tetrabutylammonium bromide aqueous solution-methanol is taken as a mobile phase, detection wavelength is 210-310 nm,column temperature is 25-40 DEG C, and mobile phase velocity is 0.5-2.0 mL / min, wherein the tetrabutylammonium bromide aqueous solution is adjusted to a pH of 8.0-9.0 with phosphoric acid. Sample solution preparation is to prepare solutions containing the Raltitrexed and the purities of the Raltitrexed of 0.1-1.0 mg / mL by using a polar solvent. The determination is to inject the solutions into high performance liquid chromatograph, record chromatograms and analyze the chromatograms. The method of the invention can be used to rapidly and accurately perform quantitative analysis on related substances of Raltitrexed bulk medicine and preparations of the Raltitrexed, thereby ensuring the quality controllability of the Raltitrexed.
Owner:NANJING CHIA TAI TIANQING PHARMA
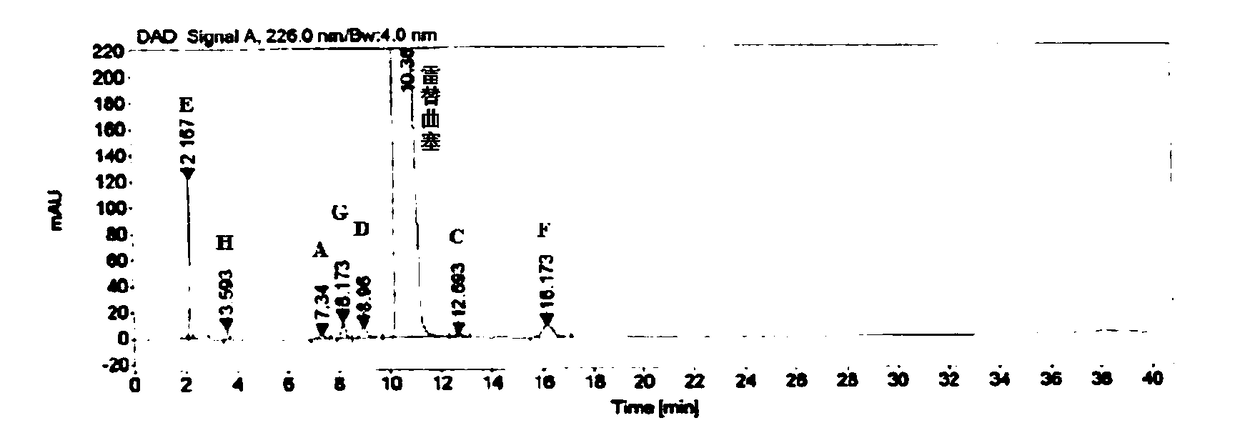
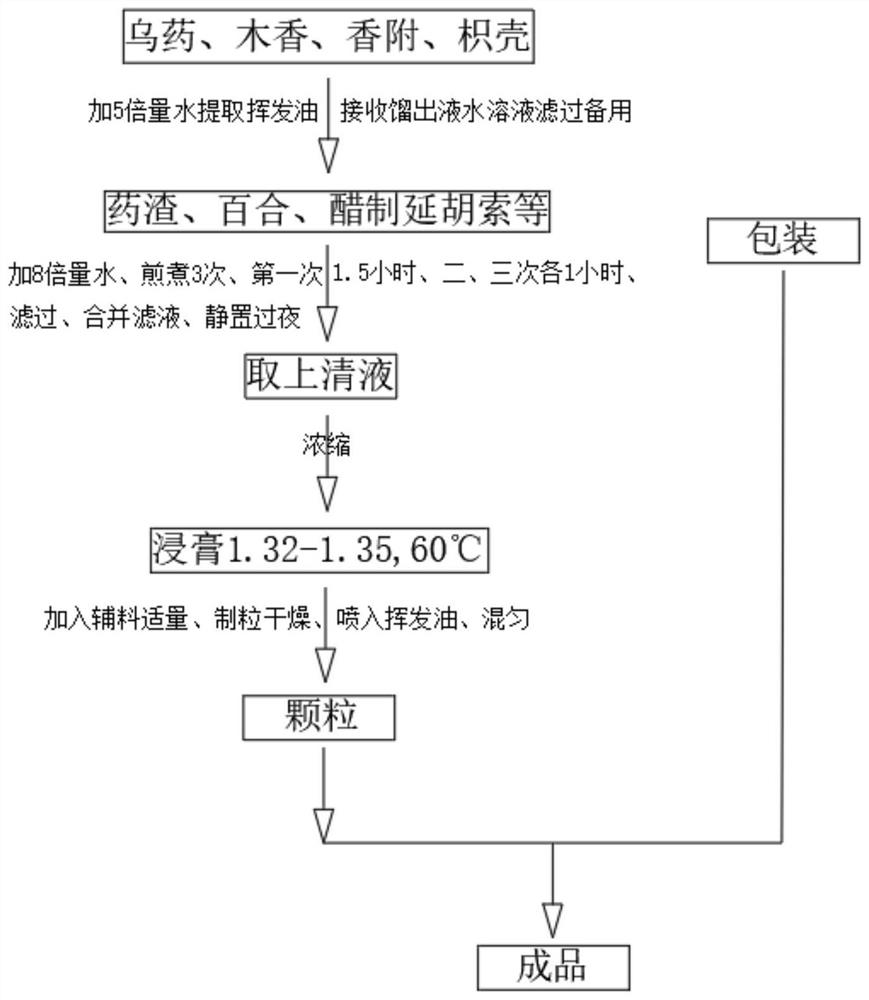
Six-in-one anti-reversion particles and preparation method thereof
InactiveCN111840457AIt has the effect of promoting qi and relieving painAntiemeticDigestive systemGranular deliveryEsophagitisLindera aggregata
The invention provides six-in-one anti-reversion particles and a preparation method thereof. The six-in-one anti-reversion particles are prepared from the following components in parts by weight: 600gof lily, 200 g of lindera aggregata, 200g of radix aucklandiae, 300g of vinegar-processed rhizoma corydalis, 300g of rhizoma cyperi, 180g of coptis chinensis, 60g of fructus evodiae and 240g of fructus aurantii. The invention also provides the preparation of the six-in-one anti-reversion particles. The preparation method comprises the following steps: S1) adding 5 times of water into the radix linderae, the radix aucklandiae, the rhizoma cyperi and the fructus aurantii, distilling for 3 hours, receiving distillate, standing, taking out a volatile oil layer for later use, and filtering a distilled water solution to obtain filtrate and medicine residues; S2) mixing the decoction dregs, lily, vinegar processed corydalis tuber, coptis chinensis and fructus evodiae, adding 8 times of water, decocting for three times, then filtering, combining filtrates, and standing overnight; and S3) taking out the supernatant of the combined filtrate. The six-in-one anti-reversion particles have a remarkable clinical effect on liver-stomach stagnated heat type esophagitis, have no recurrence and no side effect after drug withdrawal, and are convenient to popularize and use.
Owner:贵州中医药大学第一附属医院
Method for separating and determining glipizide and impurities thereof by liquid chromatography
ActiveCN111679027AAchieve complete separation at the same timeAccurate quantitative analysisComponent separationPyrazinePhosphoric acid
The invention discloses a method for separating and determining glipizide and impurities thereof through high performance liquid chromatography and application. According to the determination method,a chromatographic column taking octadecylsilane chemically bonded silica as a filler is adopted; detection conditions are as follows: chromatographic conditions: the chromatographic column is an octadecylsilane chemically bonded silica chromatographic column; the mobile phase is 0.010 to 0.018 mol / L monopotassium phosphate-acetonitrile-methanol, and the pH value is adjusted to 3.5 to 4.0 by usingphosphoric acid; the column temperature is 20 to 30 DEG C; the sample injection amount is 15 to 30 mu L; the sample injection concentration is 0.0003 to 2 mg / mL; the flow rate is 0.8 to 1.2 mL / min; and the detection wavelength is 225 nm, and gradient elution is carried out. The method disclosed by the invention is high in precision, good in repeatability and high in recovery rate, and can be widely applied to quality detection of glipizide bulk drugs with different sources and corresponding preparations of the glipizide bulk drugs.
Owner:GUANGDONG HUANAN PHARMACEUTICAL GROUP CO LTD +2
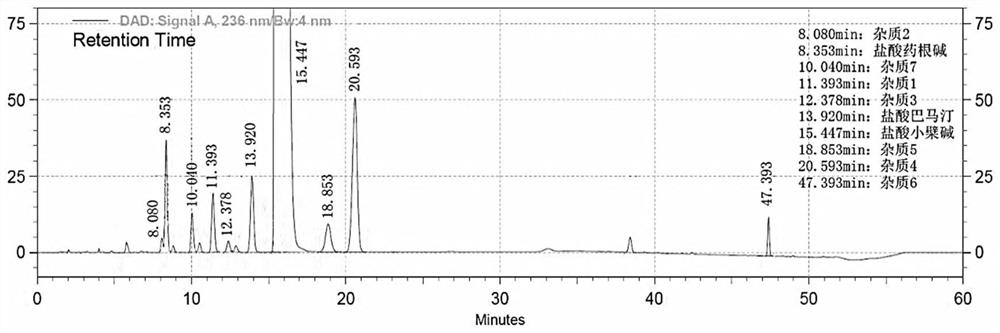
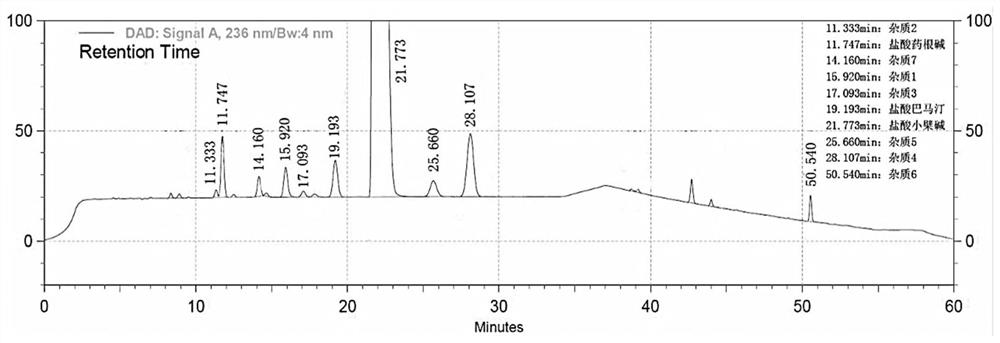
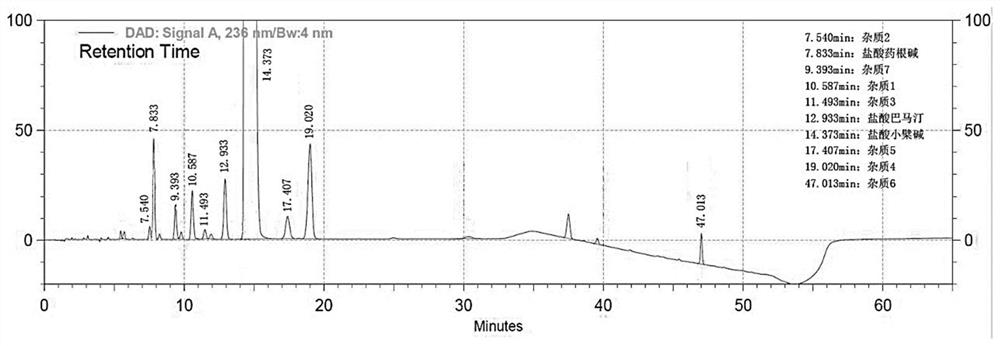
Method for separating and determining berberine and impurities thereof by liquid chromatography
ActiveCN111610272AControl impurity contentAchieve complete separation at the same timeComponent separationSilanesSilica gel
The invention discloses a method for separating and determining berberine and impurities thereof through high performance liquid chromatography and application. According to the determination method,a chromatographic column taking octadecylsilane chemically bonded silica as a filler is adopted; detection conditions are as follows: chromatographic conditions: the chromatographic column is an octadecylsilane chemically bonded silica chromatographic column, the mobile phase is 0.03-0.08 mol / L ammonium dihydrogen phosphate-acetonitrile, the pH value of the mobile phase is 3.0-3.5, the column temperature is 23-28 DEG C, the sample injection amount is 5-15 microliters, the sample injection concentration is 0.0005-2 mg / mL, the flow rate is 0.8-1.2 mL / min, the detection wavelength is 236 nm, andgradient elution is performed. The method is high in precision, good in repeatability and high in recovery rate, and can be widely applied to quality detection of berberine bulk drugs from different sources and corresponding preparations thereof.
Owner:GUANGDONG HUANAN PHARMACEUTICAL GROUP CO LTD +1
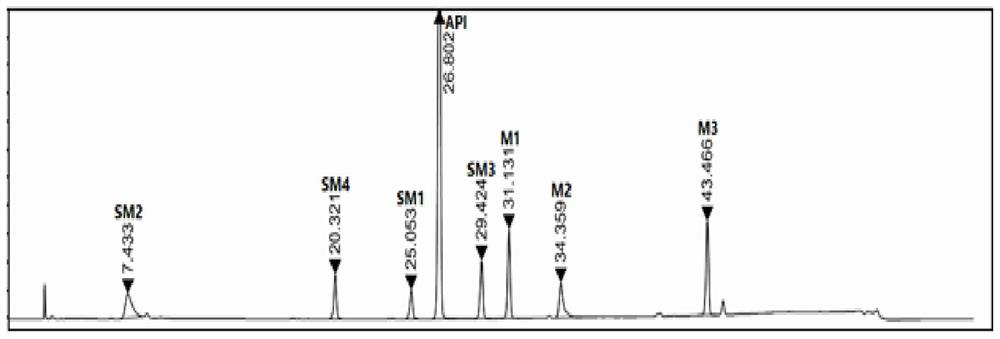
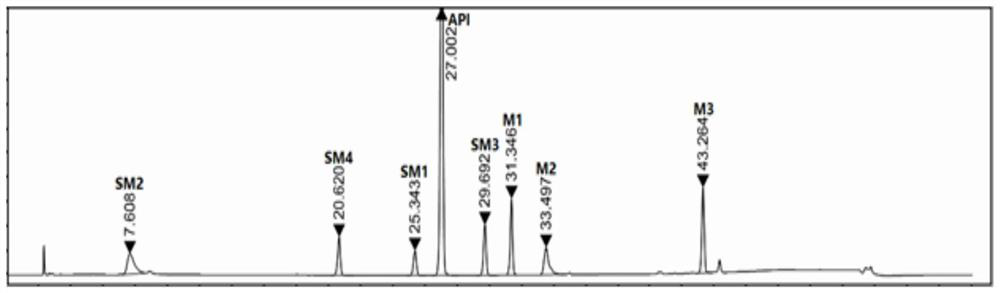
A kind of analysis method of elagolix sodium raw material and its synthetic intermediate
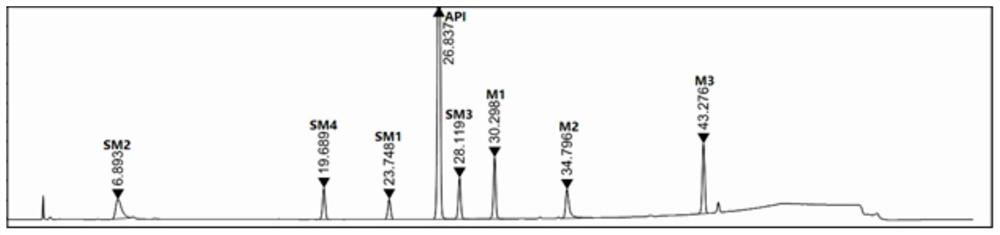
ActiveCN110501446BImprove responsivenessGood symmetryComponent separationUltraviolet detectorsFluid phase
The invention discloses a method for analyzing the raw material of elagolix sodium and its synthetic intermediate, which is characterized in that the raw material of elagolix sodium and its synthetic intermediate are analyzed by high-performance liquid chromatography. Need testing solution is elagolix sodium, the mixed solution of starting material SM1, SM2, SM3, SM4 and intermediate M1, M2, M3; Select Kromasil-Eternity-5-C18 chromatographic column, with phosphate buffer solution and Acetonitrile was used as the mobile phase, and an ultraviolet detector was selected with a detection wavelength of 200-230nm to analyze the raw material of elagolix sodium and the synthetic intermediate. It solves the problem of separation and detection of elagolix sodium and its synthetic intermediates, and ensures the quality controllability of elagolix sodium raw materials or preparations.
Owner:江苏海岸药业有限公司
Method for separating and determining carbocisteine and impurities thereof by liquid chromatography
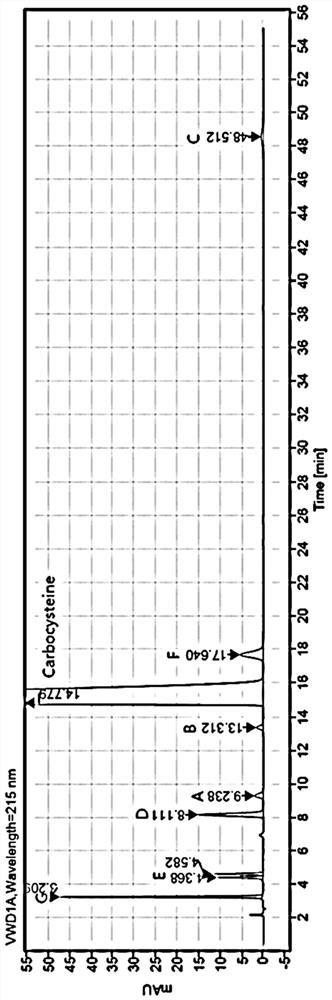
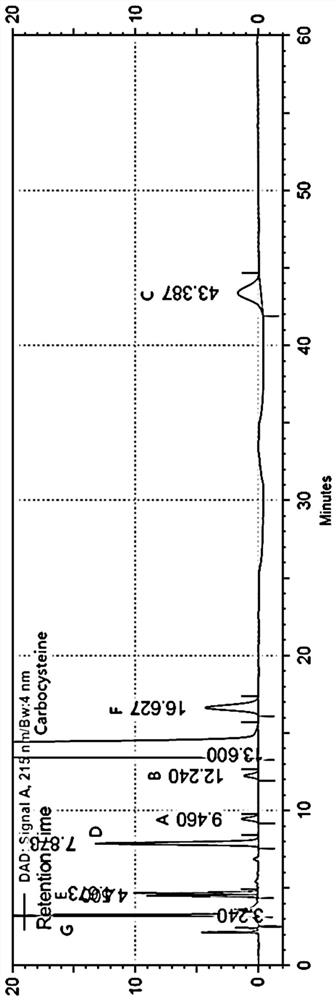
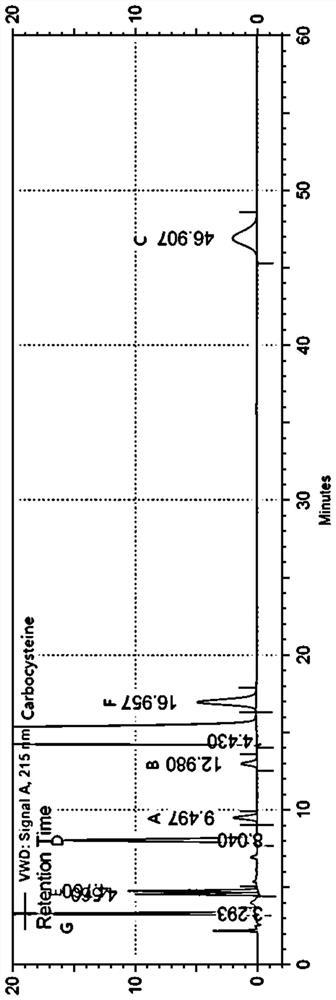
ActiveCN112782327AAchieve complete separation at the same timeAccurate quantitative analysisComponent separationFluid phaseCarbocisteine
The invention discloses a method for separating and determining carbocisteine and impurities thereof by high performance liquid chromatography. The determination method adopts a chromatographic column taking octadecylsilane chemically bonded silica as a filler, and the detection conditions are as follows: chromatographic conditions: the chromatographic column is an octadecylsilane chemically bonded silica chromatographic column, a mobile phase is a phosphate-ion pair buffer solution, the pH value of the mobile phase is 1.6-2.0, and the detection wavelength is 215 nm. The method disclosed by the invention is high in precision, good in repeatability and high in recovery rate, and can be widely applied to quality detection of carbocisteine raw material medicines from different sources and corresponding preparations thereof.
Owner:广东逸舒制药股份有限公司 +3
A method for the separation and determination of 1,2-propanediol enantiomers by gas chromatography
ActiveCN105738533BImprove smearingEnsure quality controllabilityComponent separationEnantiomerVapor phase chromatography
The invention discloses a method for separating and determining 1,2-propylene glycol, bulk drugs containing 1,2-propylene glycol and 1,2-propylene glycol enantiomer impurities in preparations of the bulk drugs by using precolumn derivatization gas chromatography. The method employs an aldehyde ketone compound as a derivative reagent, carries out precolumn derivatization on the aldehyde ketone compound and 1,2-propylene glycol in the presence of a catalyst and a water reducer and determines trace enantiomer impurities by using gas chromatography. The method provided by the invention overcomes the defect of incapability of accurate quantification of 1,2-propylene glycol enantiomer impurities due to serious trailing of peaks during frequently-used chiral gas chromatographic column separation and guarantees that the quality of 1,2-propylene glycol, the bulk drugs containing 1,2-propylene glycol and the preparations thereof is controllable.
Owner:CHONGQING PHARMA RES INST
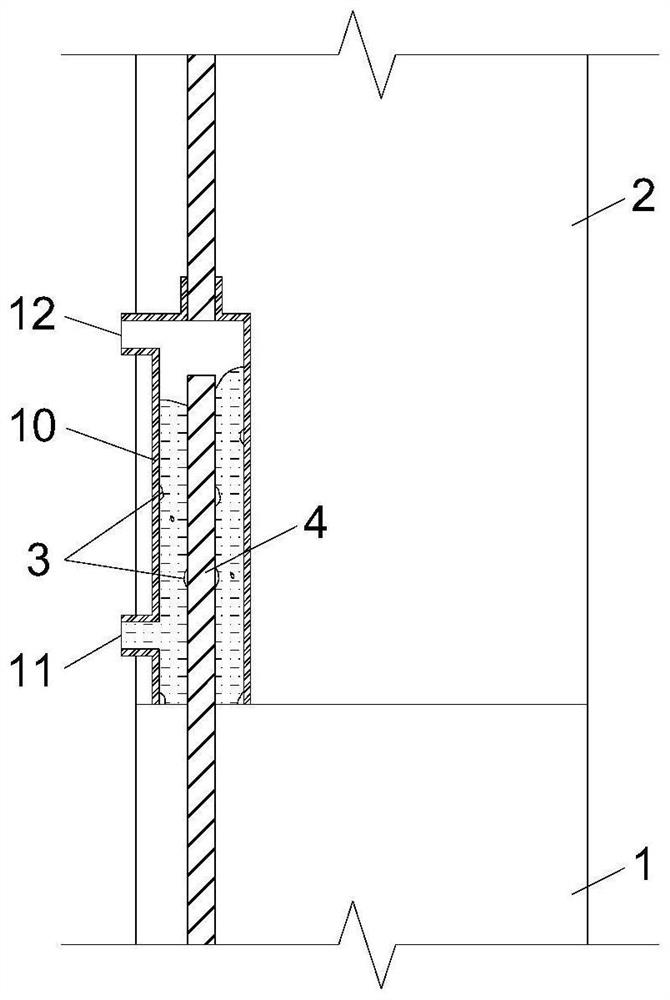
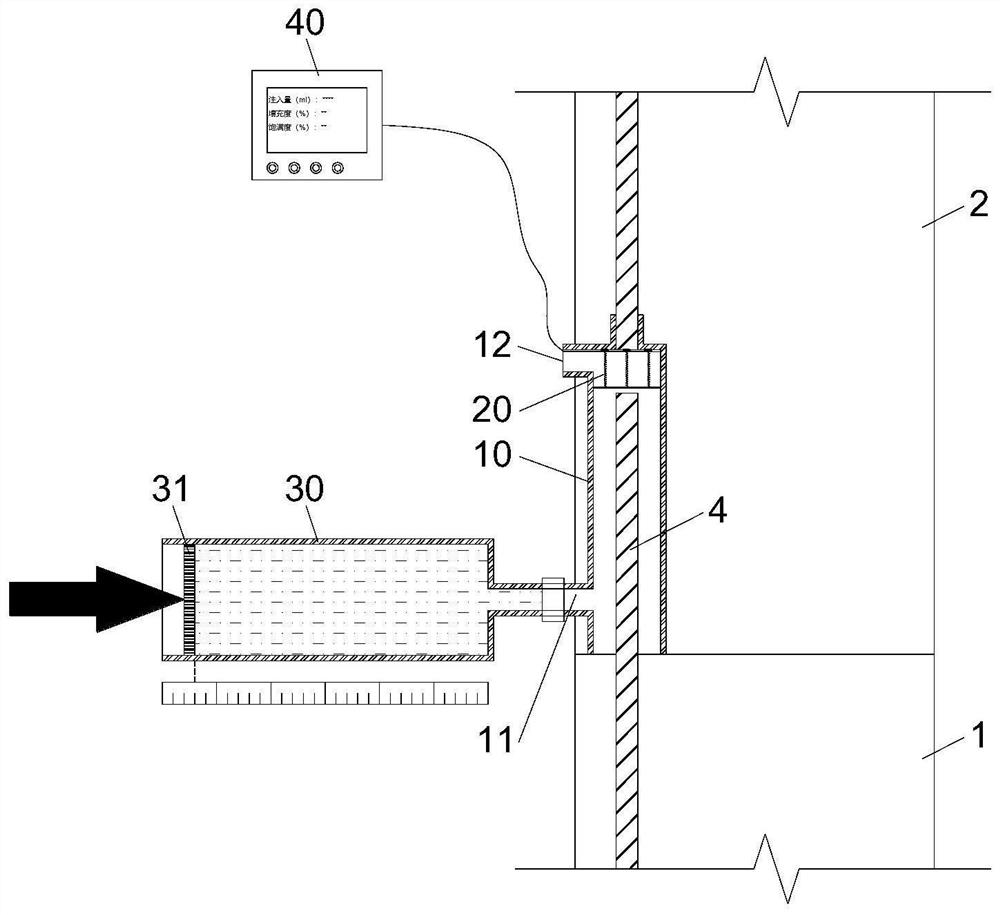
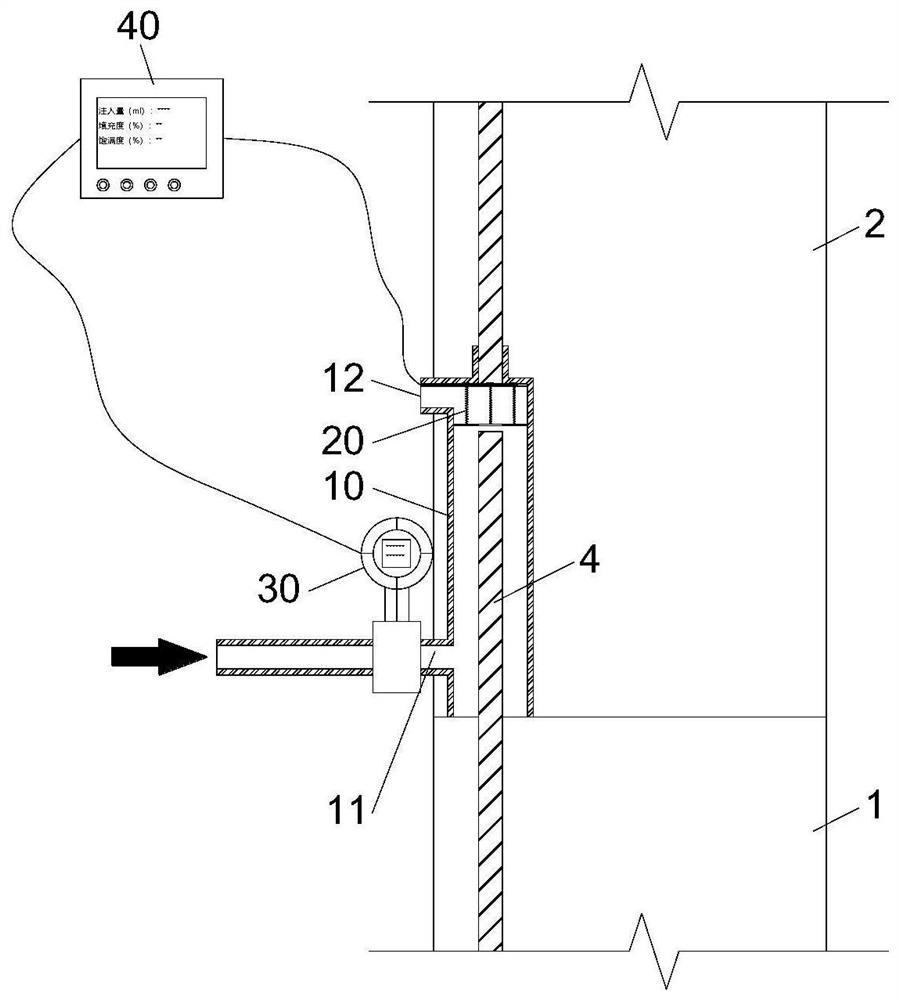
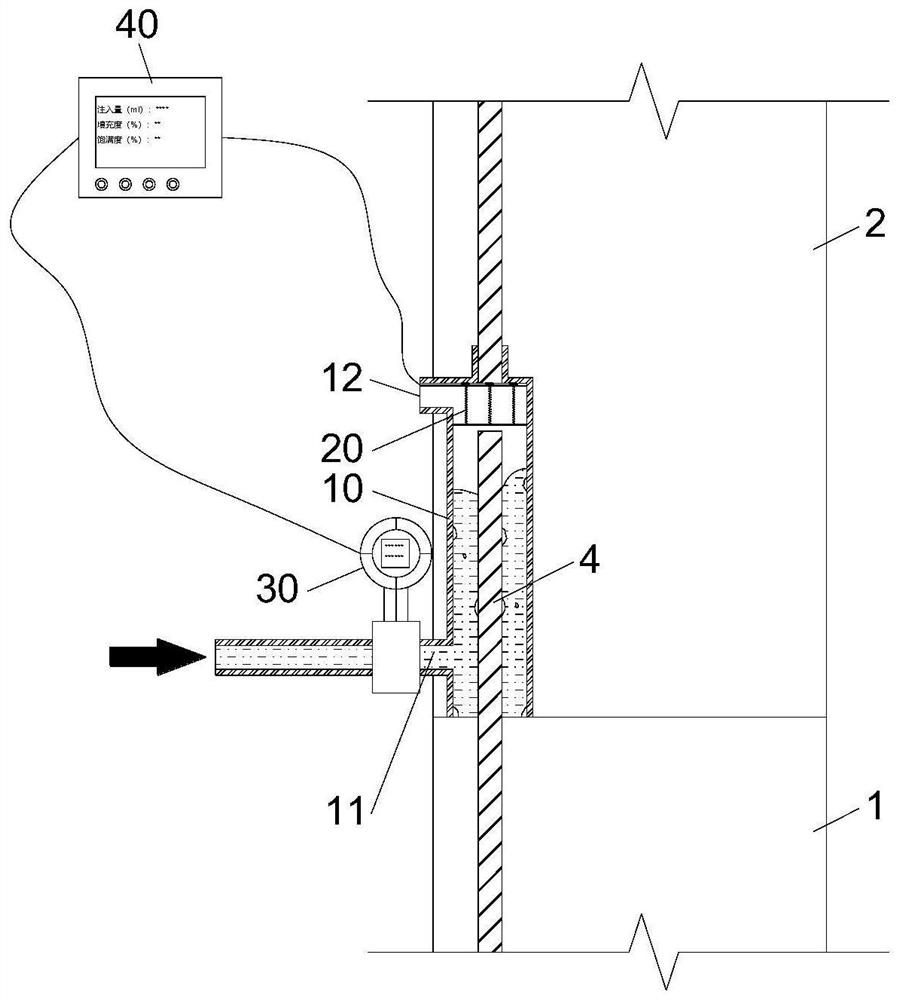
Device and method for detecting slurry filling fullness in mechanical sleeve grouting construction stage
ActiveCN112014548AEnsure quality controllabilityGood for mass productionHydro energy generationMaterial testing goodsData acquisitionSlurry
The invention discloses a mechanical sleeve grouting construction stage slurry filling fullness detection device and method, and relates to the technical field of prefabricated building structure construction quality detection. The method aims at solving the problems that an existing slurry filling fullness detection method is carried out after sleeve grouting is completed and slurry is hardened,and grouting quality defects cannot be overcome. The detection device comprises a back pressure device arranged in an inner cavity of a sleeve and a data acquisition and analysis module in signal connection with the back pressure device, and a pressure measuring plate and a back pressure piece of the back pressure device are movably connected through a plurality of suspension rods evenly distributed in the radial direction. When the slurry liquid level in the sleeve is in contact with and extrudes the back pressure sheet, the data acquisition module acquires the internal force of the sliding spring through a pressure sensor arranged at the top of the pressure measuring plate; and the filling degree, the filling plumpness and the voidage in the sleeve are calculated such that the quantitative evaluation of the actual grouting construction quality in the sleeve is completed in the construction stage, components are separated, the sleeve is cleaned and re-constructed before the slurry isnot completely condensed, and the controllability of the grouting construction quality is ensured.
Owner:SHANGHAI CONSTRUCTION GROUP
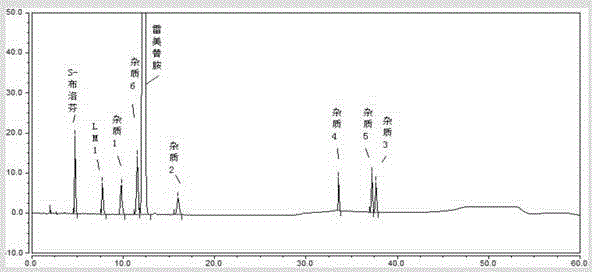
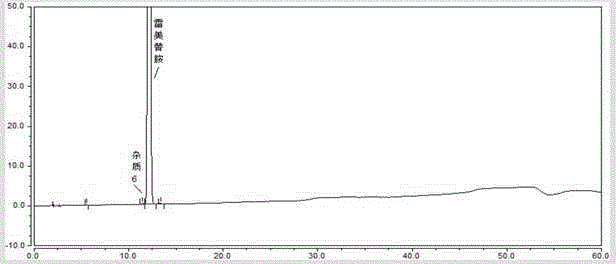
Method for determining ramelteon and impurities thereof through high-performance liquid chromatography separation
ActiveCN105277628AAchieve separationAccurate quantitative analysisComponent separationPhosphoric acidColumn temperature
The present invention discloses a method for determining ramelteon and impurities thereof through high-performance liquid chromatography separation. The technical scheme comprises that: the chromatographic conditions comprise that octadecylsilane bonded silica gel is adopted as a filler, 0.1% triethylamine solution-acetonitrile is adopted as a mobile phase, the detection wavelength is 210-310 nm, the column temperature is 20-45 DEG C, the mobile phase velocity is 0.5-2.0 ml / min, and the triethylamine solution is formed by adjusting the pH value of triethylamine with a volume fraction of 1% to 3.0-7.5 with phosphoric acid; the sample solution preparation comprises using a polar solvent to respectively prepare solutions respectively containing 0.01-2.0 mg / ml of ramelteon and 0.01-2.0 mg / ml of impurities; and the determination comprises: injecting the solution into a high-performance liquid chromatograph, recording the chromatogram, and analyzing. With the method of the present invention, the quantitative analysis can be performed on the related substances of the bulk drug ramelteon and the preparation thereof so as to ensure the quality controllability of the ramelteon and the preparation thereof.
Owner:NANJING CHANGAO PHARMA SCI & TECH CO LTD
Camptothecin-analogue crystal forms and preparing method and application thereof
InactiveCN106543195AHigh crystal stabilityGood crystal stabilityOrganic active ingredientsOrganic chemistry methodsCamptothecin derivativeCondensed matter physics
The invention provides camptothecin-analogue crystal forms and a preparing method and application thereof. The crystal forms include the crystal form A and the crystal form B, wherein in the crystal form A, Cu-K alpha radiation is adopted, and an X-ray powder diffraction spectrum of the crystal form A is characterized by comprising characteristic diffraction peaks at the portions corresponding to 2theta values of 6.95+ / -0.2 degrees, 10.35+ / -0.2 degrees, 15.15+ / -0.2 degrees, 15.85+ / -0.2 degrees and 23.25+ / -0.2 degrees; the crystal form B comprises characteristic diffraction peaks at the portions corresponding to 2theta values of 7.70+ / -0.2 degrees, 10.55+ / -0.2 degrees, 11.20+ / -0.2 degrees, 12.00+ / -0.2 degrees and 19.50+ / -0.2 degrees. The crystal form A has the quite-high crystal form stability, and the crystal form B has the good crystal form stability.
Owner:SICHUAN SINO INNOVATION BIOTECH CO LTD
Method for detecting three potential genotoxic impurities in meropenem
PendingCN114324637AEnsure safetyEnsure quality controllabilityComponent separationAgainst vector-borne diseasesMethylanilineMeropenem
The invention relates to a method for detecting three potential genotoxic impurities in meropenem, and belongs to the technical field of pharmaceutical analysis, the potential genotoxic impurities comprise 4-nitrotoluene, p-methylaniline and 4-nitrobenzyl alcohol; high performance liquid chromatography is adopted, and chromatographic conditions are as follows: an octadecyl silica gel chromatographic column; carrying out isocratic elution by taking a monopotassium phosphate buffer solution and methanol as mobile phases; the ultraviolet detector adopts a dual-wavelength mode; the flow rate is 0.5-2.0 ml / min, the sample injection volume is 10-100 microliters, and the column temperature is 20-50 DEG C; during determination, a system applicability solution, an impurity positioning solution and a test solution are respectively injected into a high performance liquid chromatograph, and chromatograms are recorded. According to the method, three genotoxic impurities in meropenem are controlled at the same time through the high performance liquid chromatography, complete separation of all the impurities is achieved, operability and specificity are high, and high sensitivity, accuracy and precision are achieved.
Owner:深圳华药南方制药有限公司
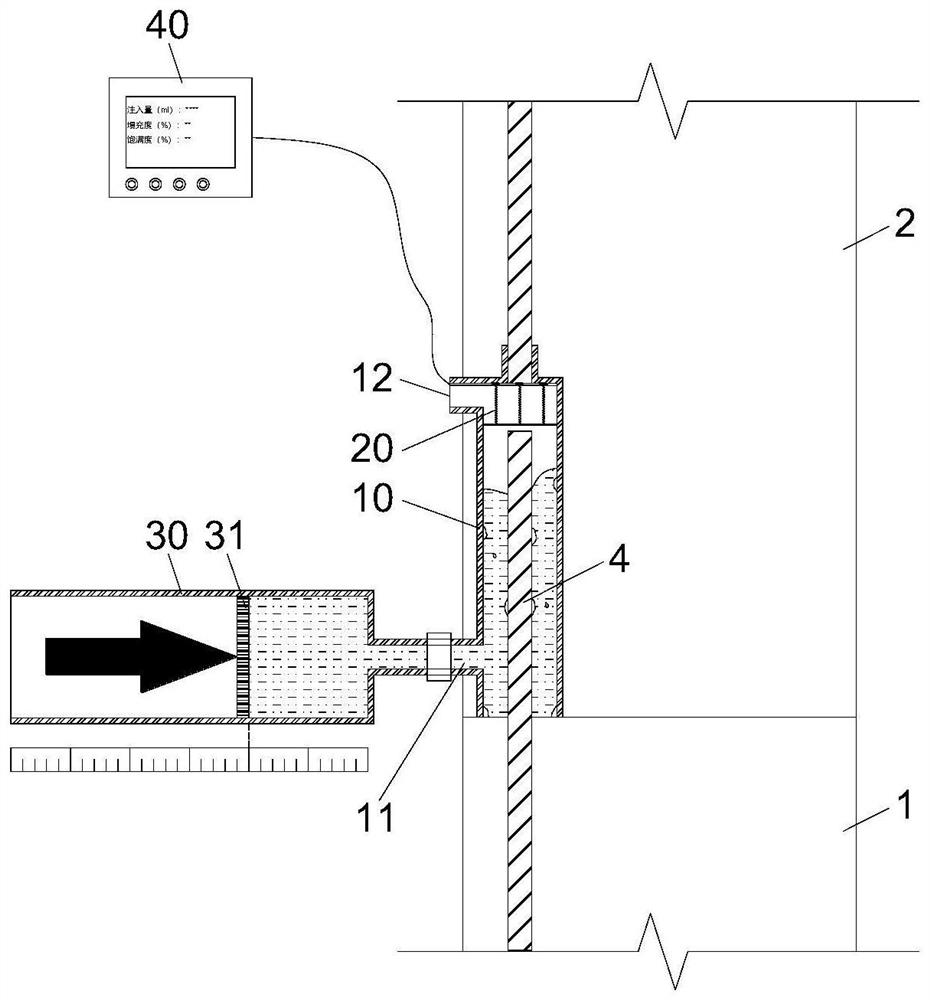
Device and method for detecting slurry filling fullness in electromagnetic sleeve grouting construction stage
The invention discloses a device and method for detecting slurry filling fullness in an electromagnetic sleeve grouting construction stage, and relates to the technical field of prefabricated buildingstructure construction quality detection. The method aims at solving the problems that an existing slurry filling fullness detection method is carried out after sleeve grouting is completed and slurry is hardened, and grouting quality defects cannot be overcome. The detection device comprises a back pressure device arranged in an inner cavity of the sleeve and a data acquisition and analysis module in signal connection with the back pressure device, a base of the back pressure device is movably connected with a back pressure piece through a plurality of sliding rods evenly distributed in theradial direction, the sliding rods are sliding rheostats with resistors evenly distributed in the length direction of the sliding rods, and each sliding rod is provided with a sliding spring to provide back pressure. The liquid level of slurry in the sleeve makes contact with and extrudes the back pressure piece to slide upwards along the sliding rods, the data collecting and analyzing module measures the resistance value of each sliding rod through the resistance measuring device at present, then the filling degree, the filling plumpness and the voidage in the sleeve are calculated, and thusquantitative evaluation of the actual grouting construction quality in the sleeve is completed in the construction stage.
Owner:SHANGHAI CONSTRUCTION GROUP
A method for analyzing obeticholic acid and its synthetic intermediates by high performance liquid chromatography
ActiveCN107917972BHigh detection sensitivityGuaranteed stabilityComponent separationCholic acidPhosphate
Owner:JIANGSU SKYRUN PHARMA CO LTD
Quantitative determination method for genotoxic impurities in calcium dobesilate
PendingCN114839293AAchieve separationMild reaction conditionsComponent separationOther chemical processesSilica gelDerivatization
The invention discloses a quantitative determination method of genotoxic impurities in calcium dobesilate, which comprises the following steps: firstly, preparing a derivatization reagent by adopting an acidic acetonitrile solution, so that stable existence of calcium dobesilate, 2-sulfonyl-1, 4-benzoquinone, hydroquinone and 1, 4-benzoquinone can be ensured, and meanwhile, complete reaction of benzoquinone impurities with the derivatization reagent can be ensured; and fully dissolving the sample and impurities by using an acetonitrile aqueous solution. Aiming at the derivatized sample solution, the separation and quantification of the benzoquinone impurities are realized under the high performance liquid chromatography conditions that a chromatographic column taking terminated octadecylsilane chemically bonded silica as a filling agent is adopted, and a mixed solution of a buffer solution and acetonitrile with the pH value of 4.5 + / -0.5 is adopted as a mobile phase. The method is convenient to operate, good in detection specificity of the benzoquinone impurities in the sample, high in sensitivity, good in precision and good in reproducibility, two benzoquinone impurities in calcium dobesilate can be accurately and quantitatively analyzed, and the safety and quality controllability of calcium dobesilate raw materials and preparations are guaranteed.
Owner:NANJING CHANGAO PHARMA SCI & TECH CO LTD
Detector combined method for measuring impurities of repaglinide in repaglinide metformin tablets
InactiveCN109884201AEnsure quality controllabilityHigh sensitivityComponent separationInjection volumeSilanes
The invention belongs to the field of pharmaceutical analysis. Five kinds of impurities of repaglinide in repaglinide metformin tablets are separated and measured by a technology of a high performanceliquid chromatography with two detectors in series. According to the chromatography, octyl silane bonded silica gel is used as a filling agent; monopotassium phosphate buffer solution (the mass fraction is 0.1%-1% and the pH value is 2.0-8.0)-acetonitrile is used as the mobile phase; the detectors are a diode array and a fluorescence detector in series, the detection wavelength is 210-310nm, theexcitation wavelength is 200nm-300nm, and the emission wavelength is 300-400nm; and the flow rate is 0.5-2.0ml / min, the injection volume is 10-100[mu]l, and the sample solution concentration is 0.01-1.0mg / ml. By using the method, the five kinds of the impurities of repaglinide in repaglinide metformin tablets can be calculated accurately and quantificationally, and the quality controllability of the repaglinide metformin tablets is ensured.
Owner:SHANDONG ACADEMY OF PHARMACEUTICAL SCIENCES
Ginseng-acanthopanax oral liquid and production process thereof
ActiveCN103040889BGuaranteed safetyNo "dryness"Pharmaceutical delivery mechanismAntinoxious agentsBiotechnologySyringin
Owner:四川合泰新光医药有限公司
Steel plate material tailpiece automatic identifying and dispensing system
PendingCN108480502AImprove efficiencyImproving the straight rate of qualityMetal-working feeding devicesMetal working apparatusProgrammable logic controllerControllability
A steel plate material tailpiece automatic identifying and dispensing system comprises a dispensing robot, at least one material trolley, a protecting plate collection table and a programmable logic controller (PLC) circuit; each material trolley is provided with a tailpiece detector; both the dispensing robot and the tailpiece detectors are connected with the PLC circuit; steel plates to be captured by the dispensing robot are stacked on the material trolleys, with a protecting plate being disposed under each stack of steel plate; the protecting plates are captured by the dispensing robot andput on the said protecting plate collection table; and a tailpiece detector is also arranged under the protecting plate collection table. Instead of the conventional manual monitoring method, an automatic identifying and dispensing mode is realized. In this manner, the dispensing efficiency of steel plate materials is improved, the straightness of sheet material stamped parts is promoted, and controllability of product quality is ensured.
Owner:SOUEAST
Method for separation and determination of impurities in vitamin E and its preparations by HPLC
ActiveCN107202849BEnsure quality controllabilityHigh detection sensitivityComponent separationEthylic acidGradient elution
Owner:CHINESE MEDICINES GUANGZHOU
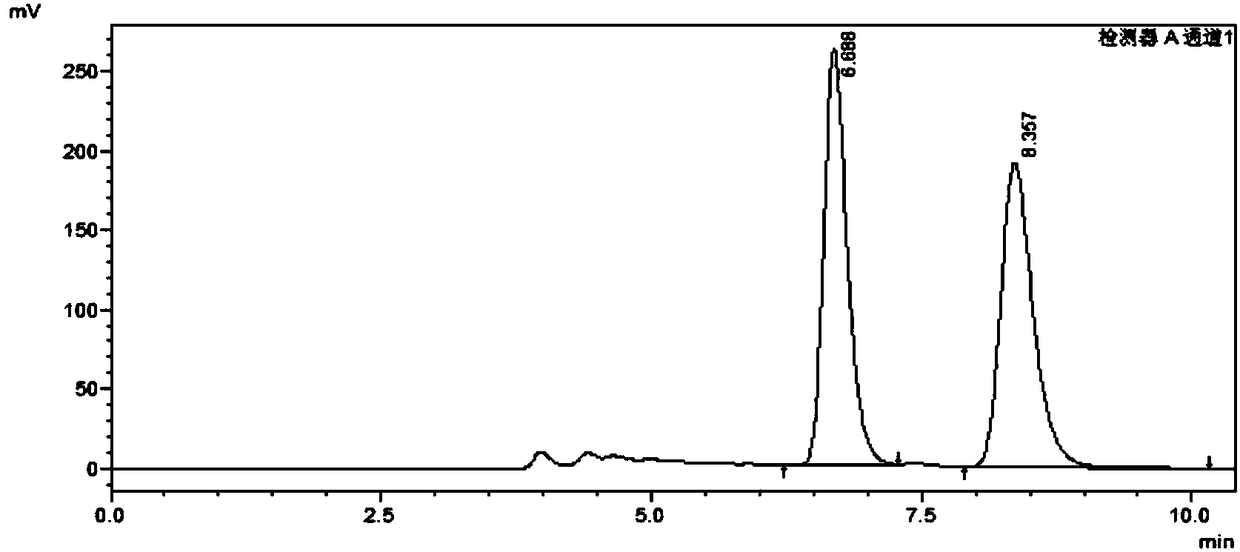
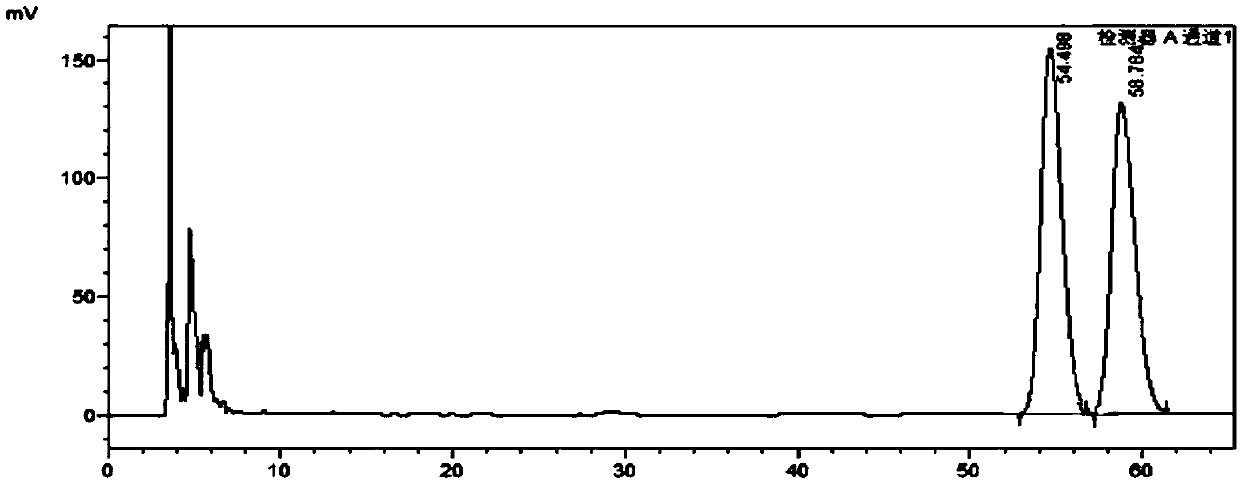
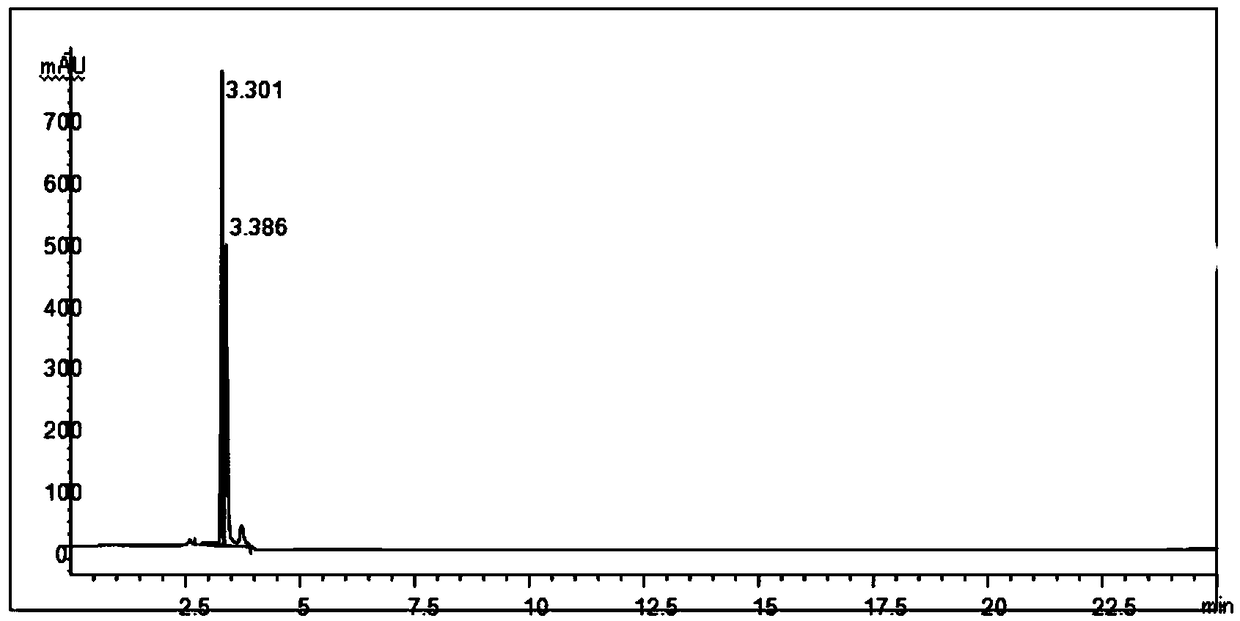
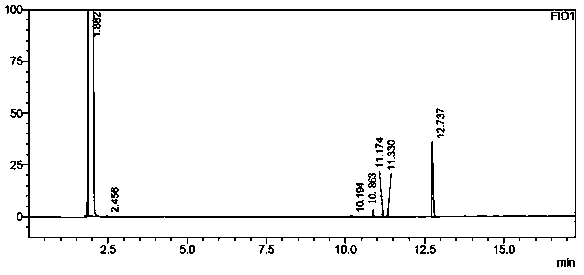
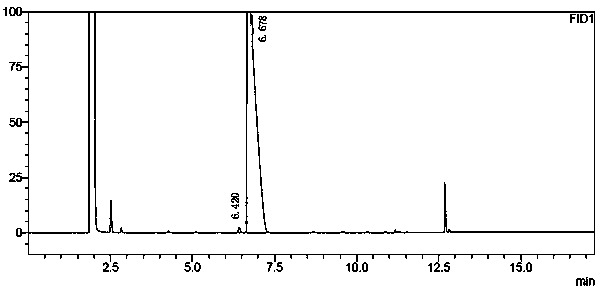
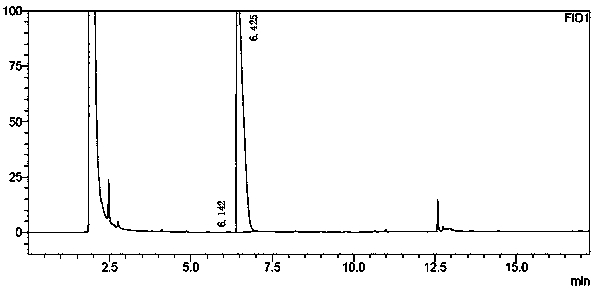
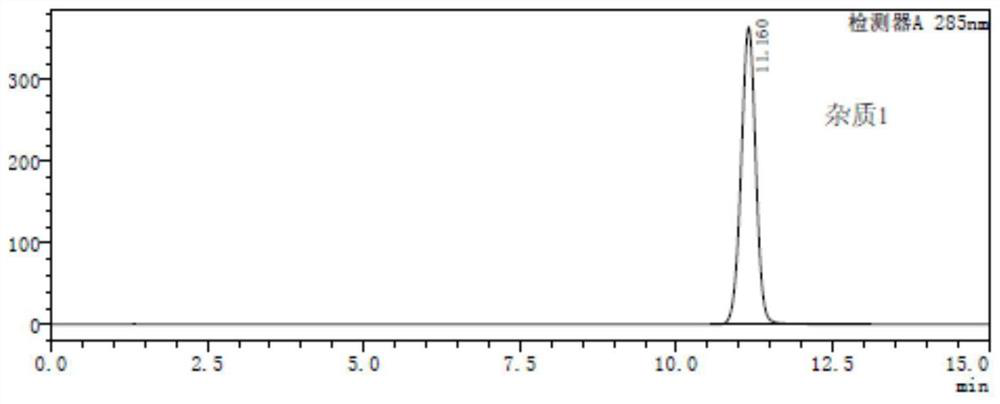
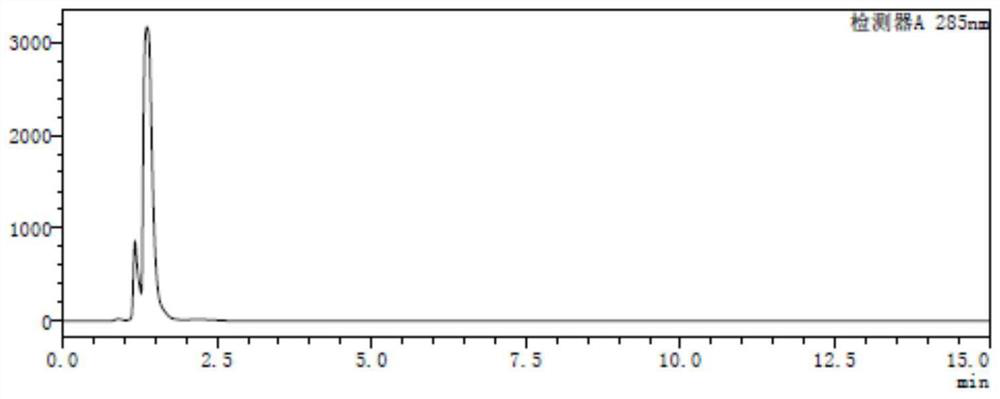
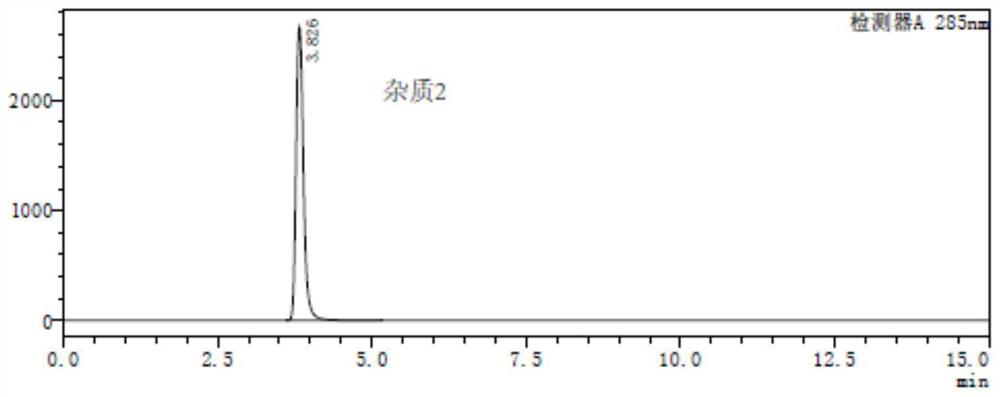
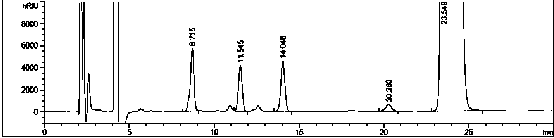
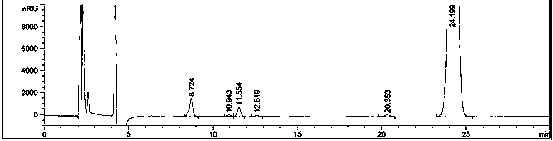
A method for analyzing and separating cis-bicyclo[3,2,0]hept-2-en-6-one enantiomers by HPLC
ActiveCN105784878BSolving Analysis Separation ProblemsControl impurity contentComponent separationHplc methodEnantiomer
Owner:CHANGCHUN BC&HC PHARMA TECH CO LTD
Thin-layer identification method for allium macrostemon test sample
ActiveCN113759066AEasy to separateMulti-layer informationComponent separationFormularyMedicinal herbs
The invention provides a thin-layer identification method of an allium macrostemon test sample, which comprises the following steps: preparing an allium macrostemon medicinal material, an aqueous extract or formula granules into a test sample solution; and dripping the test solution on a silica gel G thin-layer plate, performing developing by using petroleum ether-ethyl acetate-dichloromethane-formic acid as a developing solvent according to a volume ratio of (5-6): (3.5-4): (1-1.2): (0.8-1), taking out the silica gel G thin-layer plate, performing airing, and performing inspecting by using an ultraviolet lamp. The method has the advantages that more thin-layer chromatography information spots can be obtained compared with that in pharmacopoeia, and thin-layer information is rich; in addition, the method has the advantages that displayed spots are clear, the separation degree is good, and the repeatability is good.
Owner:BEIJING KANGRENTANG PHARMA
Method for separating and determining ramelteon and its impurities by high performance liquid chromatography
ActiveCN105277628BAchieve separationAccurate quantitative analysisComponent separationPhosphoric acidColumn temperature
The present invention discloses a method for determining ramelteon and impurities thereof through high-performance liquid chromatography separation. The technical scheme comprises that: the chromatographic conditions comprise that octadecylsilane bonded silica gel is adopted as a filler, 0.1% triethylamine solution-acetonitrile is adopted as a mobile phase, the detection wavelength is 210-310 nm, the column temperature is 20-45 DEG C, the mobile phase velocity is 0.5-2.0 ml / min, and the triethylamine solution is formed by adjusting the pH value of triethylamine with a volume fraction of 1% to 3.0-7.5 with phosphoric acid; the sample solution preparation comprises using a polar solvent to respectively prepare solutions respectively containing 0.01-2.0 mg / ml of ramelteon and 0.01-2.0 mg / ml of impurities; and the determination comprises: injecting the solution into a high-performance liquid chromatograph, recording the chromatogram, and analyzing. With the method of the present invention, the quantitative analysis can be performed on the related substances of the bulk drug ramelteon and the preparation thereof so as to ensure the quality controllability of the ramelteon and the preparation thereof.
Owner:NANJING CHANGAO PHARMA SCI & TECH CO LTD
Method for separating and determining raltitrexed and its impurities by high performance liquid chromatography
ActiveCN109283262BAchieve separationHigh detection sensitivityComponent separationPhosphoric acidColumn temperature
The invention discloses a method for separation and determination of Raltitrexed and impurities of the Raltitrexed by high performance liquid chromatography. Chromatographic conditions of the method are that octadecylsilane chemically bonded silica is taken as a filler, 0.005 mol / L tetrabutylammonium bromide aqueous solution-methanol is taken as a mobile phase, detection wavelength is 210-310 nm,column temperature is 25-40 DEG C, and mobile phase velocity is 0.5-2.0 mL / min, wherein the tetrabutylammonium bromide aqueous solution is adjusted to a pH of 8.0-9.0 with phosphoric acid. Sample solution preparation is to prepare solutions containing the Raltitrexed and the purities of the Raltitrexed of 0.1-1.0 mg / mL by using a polar solvent. The determination is to inject the solutions into high performance liquid chromatograph, record chromatograms and analyze the chromatograms. The method of the invention can be used to rapidly and accurately perform quantitative analysis on related substances of Raltitrexed bulk medicine and preparations of the Raltitrexed, thereby ensuring the quality controllability of the Raltitrexed.
Owner:NANJING CHIA TAI TIANQING PHARMA
A method for HPLC analysis and separation of enantiomers of p-phenylbenzoylcorylide
ActiveCN106198808BGuaranteed stabilityGood symmetryComponent separationEnantiomerChromatographic column
The invention discloses a method for HPLC analysis and separation of 4-phenylbenzoyl Corey lactone enantiomers; a chiral chromatographic column is adopted for analysis, separation and determination by normal phase chromatography. The method can simply, accurately and efficiently analyze and separate the 4-phenylbenzoyl Corey lactone enantiomers, so as to achieve quality control of the 4-phenylbenzoyl Corey lactone enantiomers. The method can also be used for preparation of high-purity single-opticity 4-phenylbenzoyl Corey lactone.
Owner:CHANGCHUN BC&HC PHARMA TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


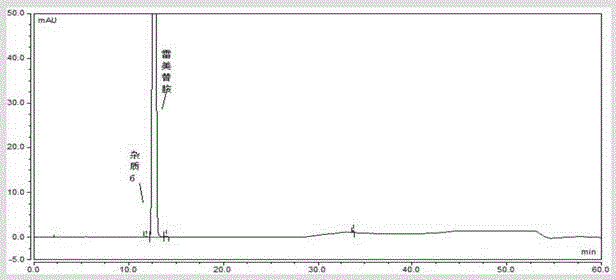
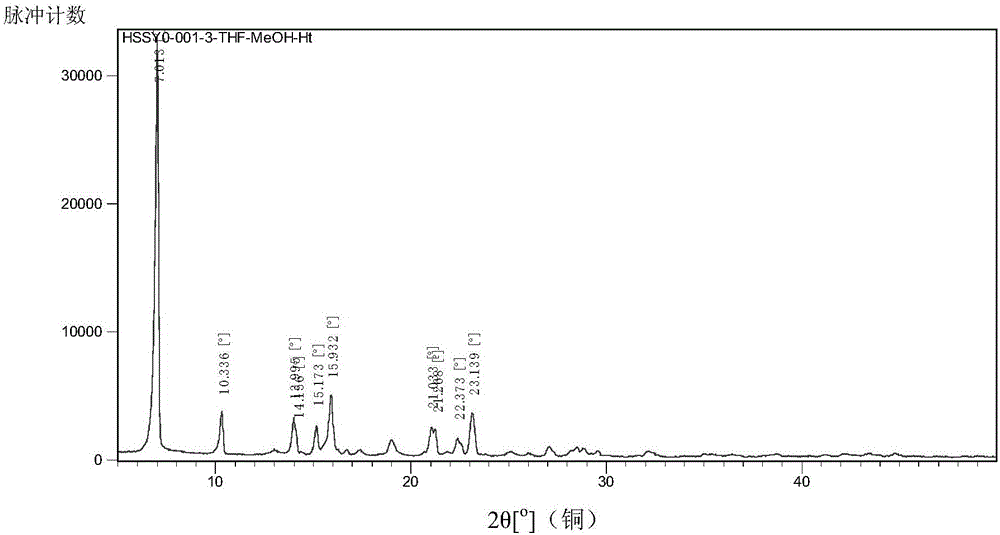
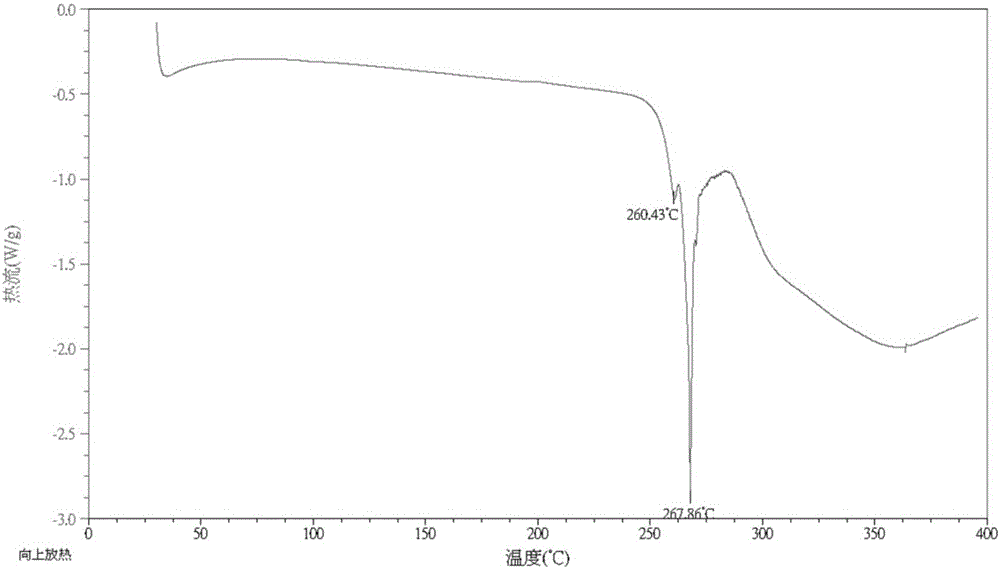
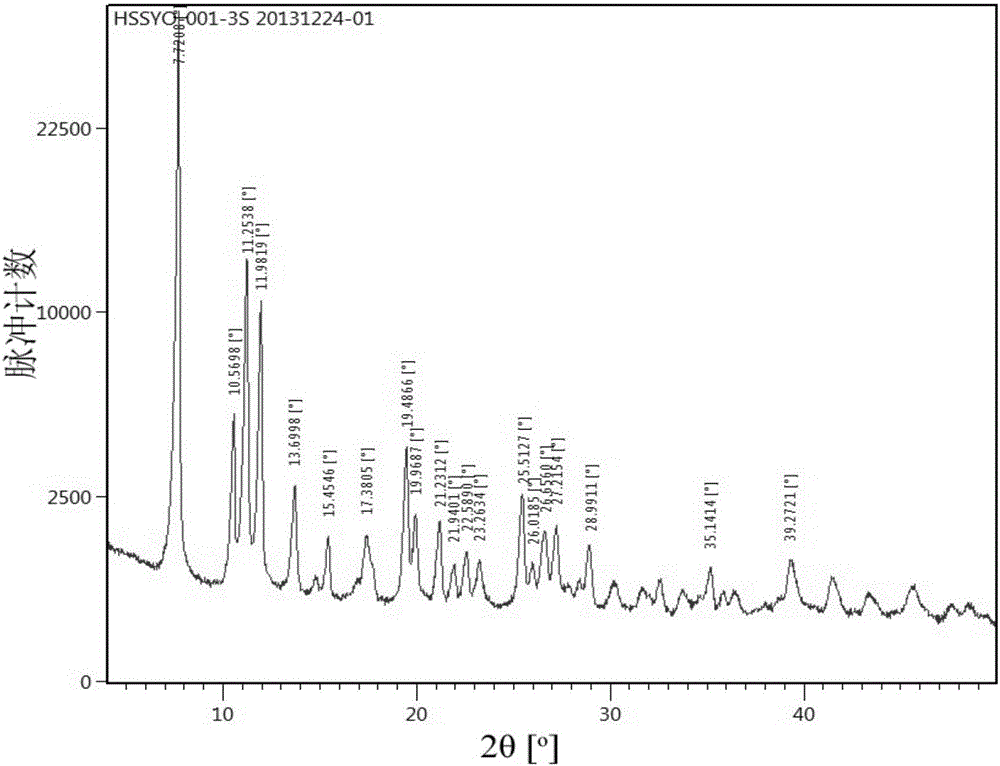

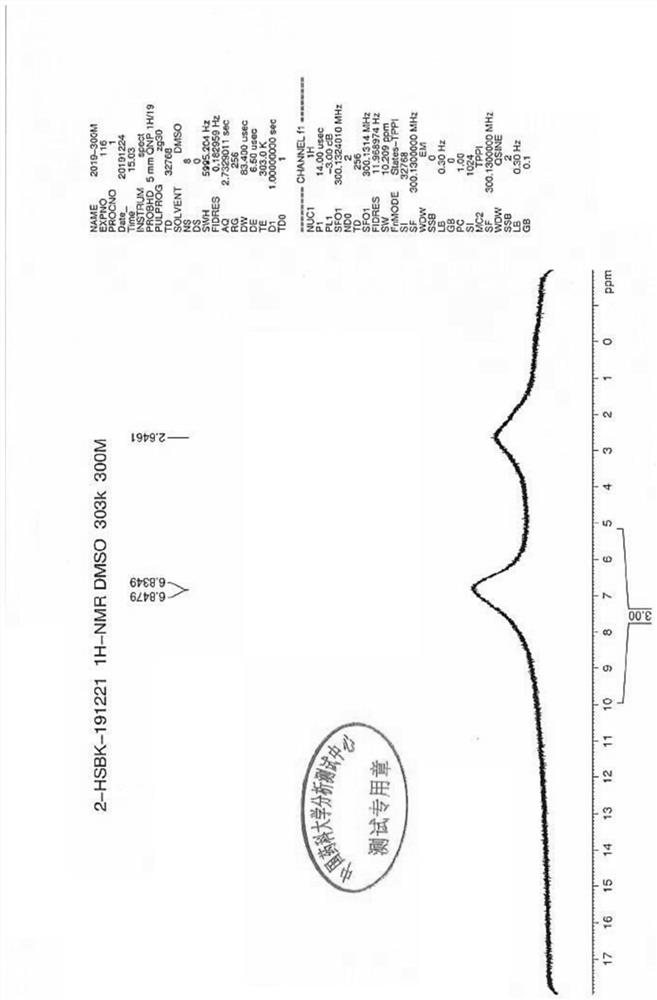
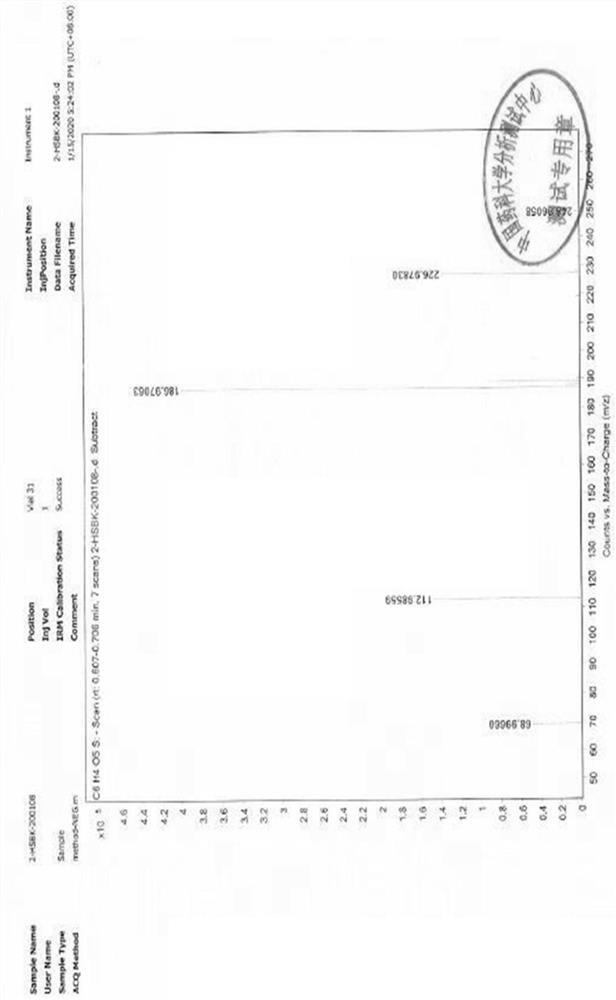

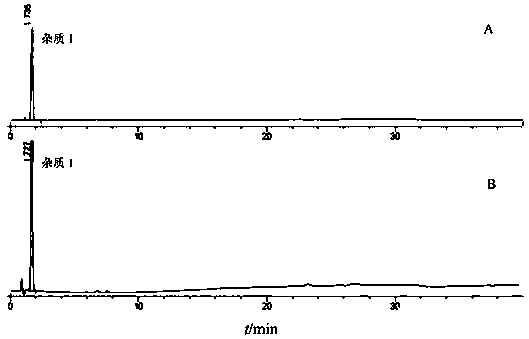
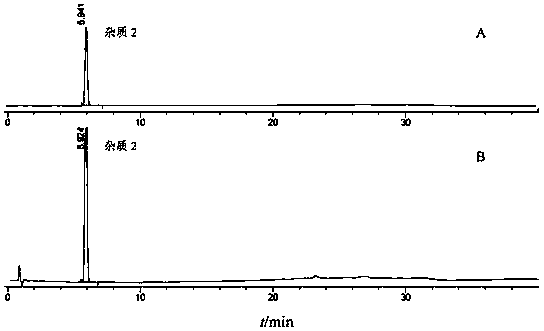
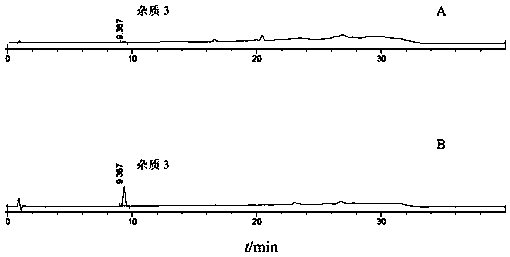
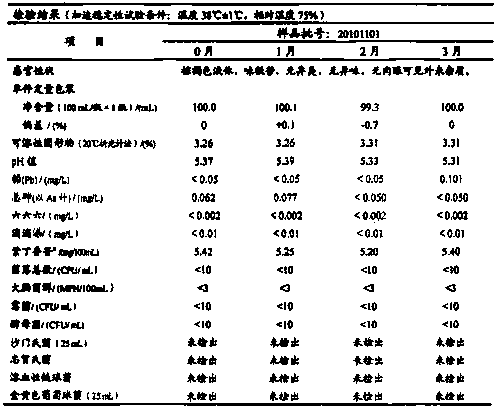
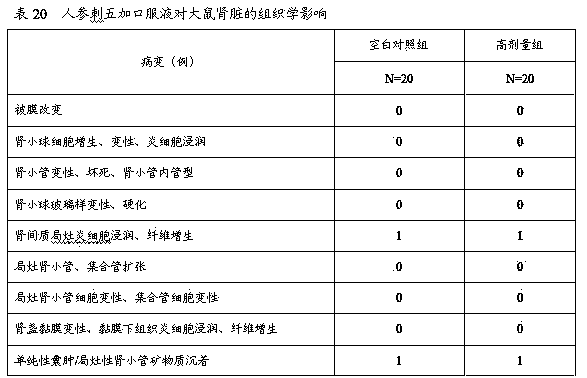
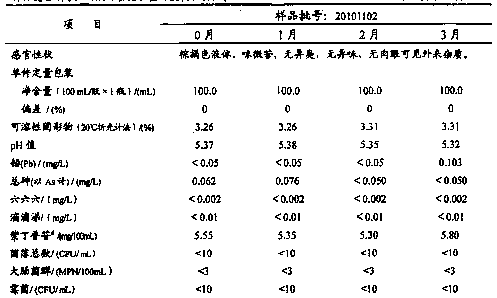
![A method for analyzing and separating cis-bicyclo[3,2,0]hept-2-en-6-one enantiomers by HPLC A method for analyzing and separating cis-bicyclo[3,2,0]hept-2-en-6-one enantiomers by HPLC](https://images-eureka.patsnap.com/patent_img/56c6164d-e766-4fef-be1a-f9dacf34be7c/BDA0000988815900000011.png)