Method for separating and determining ramelteon and its impurities by high performance liquid chromatography
A technology of high performance liquid chromatography and ramelteon, applied in the field of separation and determination of ramelteon and its impurities by high performance liquid chromatography, to achieve the effect of ensuring quality controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Instrument: Diana U3000PDA high performance liquid chromatograph;
[0045] Chromatographic column: Agilent ZORBAXSB-AQC18 chromatographic column (4.6mm×250mm, 5μm);
[0046] Mobile phase: mobile phase A is 0.1% triethylamine solution (adjust pH 6.0 with phosphoric acid), mobile phase B is acetonitrile, carry out gradient elution according to the following table:
[0047]
[0048] Column temperature: 30°C;
[0049] Flow rate: 1.0ml / min;
[0050] Detection wavelength: 230nm.
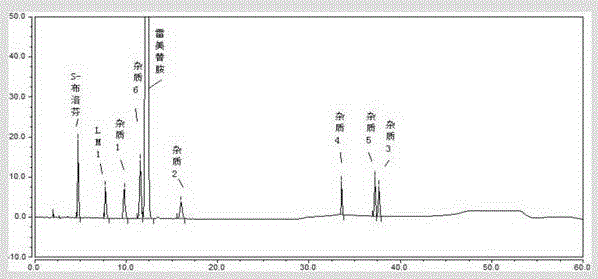
[0051] Preparation of positioning solution: take appropriate amount of LM-1, S-IBU, impurity 1, impurity 2, impurity 3, impurity 4, impurity 5, impurity 6, ramelteon reference substance, respectively add 50% acetonitrile solution to dissolve and dilute Prepare a solution containing 1mg per 1ml as a positioning solution.
[0052]The preparation of ramelteon and impurity mixed solution: get about 10mg of ramelteon test sample, accurately weighed, put in 10ml measuring bottle, add precisely 0.1m...
Embodiment 2
[0059] Instrument: Diana U3000 high performance liquid chromatography;
[0060] Chromatographic column: DikmaC18 chromatographic column (4.6mm×250mm, 5μm);
[0061] Mobile phase: mobile phase A is 0.1% triethylamine solution (adjust pH 3.0 with phosphoric acid), mobile phase B is acetonitrile, carry out gradient elution according to the following table:
[0062]
[0063] Column temperature: 30°C;
[0064] Flow rate: 1.0ml / min;
[0065] Detection wavelength: 230nm.
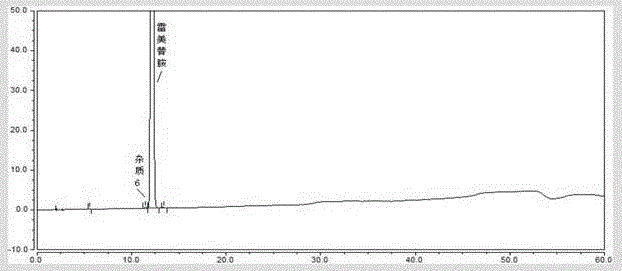
[0066] Solution preparation method and assay method are carried out according to embodiment 1, the collection of illustrative plates of ramelteon system suitability solution, ramelteon need testing solution, ramelteon sheet solution, ramelteon sheet blank excipient solution see respectively Figure 5 , 6 , 7, 8.
Embodiment 3
[0068] Instrument: Agilent1200HPLC;
[0069] Chromatographic column: Phenomenex luna-C18 chromatographic column (4.6mm×250mm, 5μm);
[0070] Mobile phase: mobile phase A is 0.1% triethylamine solution (adjust pH 7.5 with phosphoric acid), mobile phase B is acetonitrile, carry out gradient elution according to the following table:
[0071]
[0072]
[0073] Column temperature: 30°C;
[0074] Flow rate: 1.5ml / min;
[0075] Detection wavelength: 230nm.
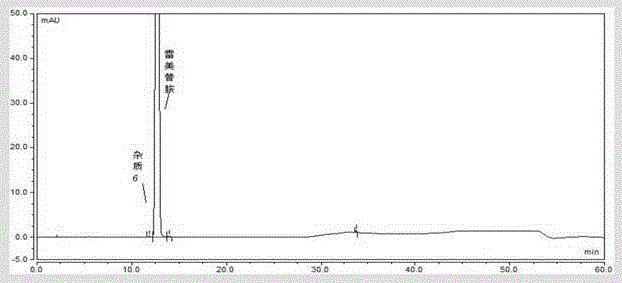
[0076] Solution preparation method and assay method are carried out according to embodiment 1, the collection of illustrative plates of ramelteon system suitability solution, ramelteon need testing solution, ramelteon tablet powder solution, ramelteon tablet blank excipient solution see respectively Figure 9 , 10 , 11, 12.
PUM
| Property | Measurement | Unit |
|---|---|---|
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com