Camptothecin-analogue crystal forms and preparing method and application thereof
A technology of crystal form and diffraction peak, which is applied in the field of crystal form of camptothecin derivatives and its preparation, can solve problems such as major adverse reactions and poor solubility of camptothecin, and achieve the effect of ensuring safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
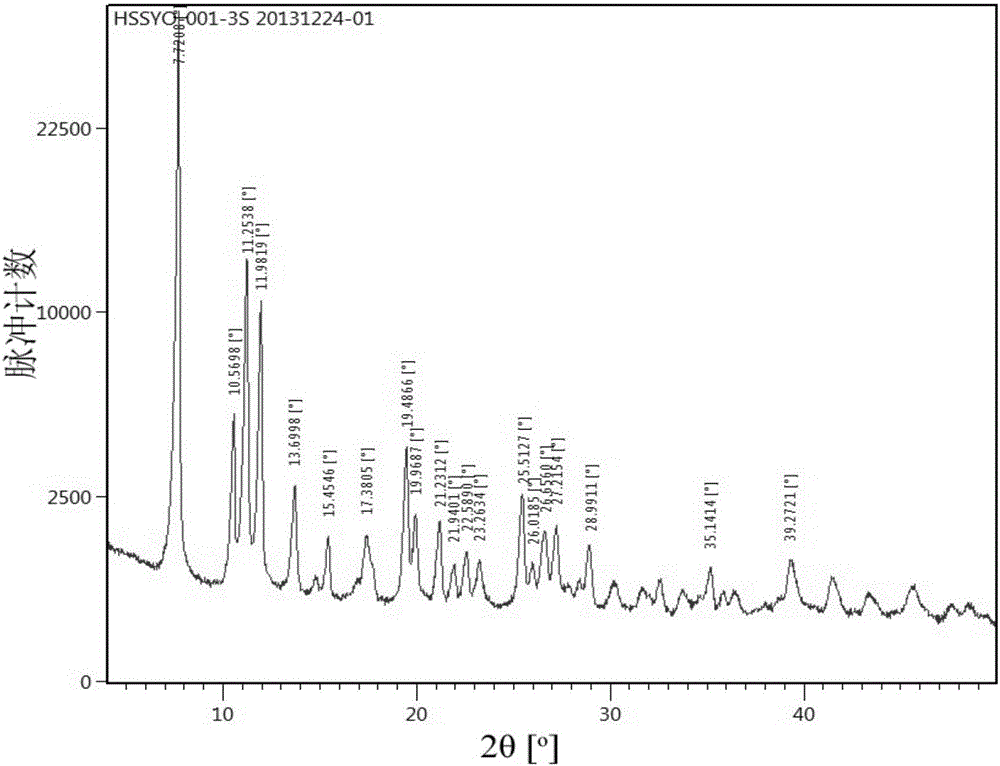
[0064] 5.5 g of starting material (S)-5-amino-4,11-diethyl-4-hydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b] Quinoline-3,14(4H,12H)dione was dissolved in 200ml of dichloromethane, evaporated to dryness under reduced pressure at 40°C, added 170mL of n-heptane, spin-dried under reduced pressure at 40°C, and added 170mL of n-heptane again The alkane was spin-dried under reduced pressure at 40°C to obtain a solid product with a yield of 100%. The number is HSSYO-001-3S 20131224-02, and its XRPD is as follows Figure 5 or its partial enlargement Image 6 shows that the obtained solid is (S)-5-amino-4,11-diethyl-4-hydroxy-1H-pyrano[3',4':6,7]indolizino[1,2 -b] Form A of quinoline-3,14(4H,12H)dione.
Embodiment 2
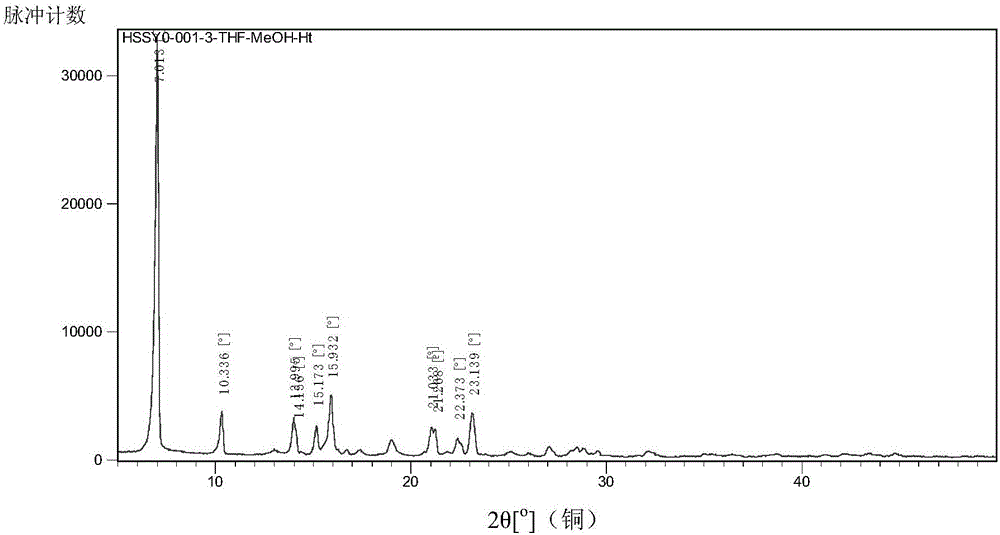
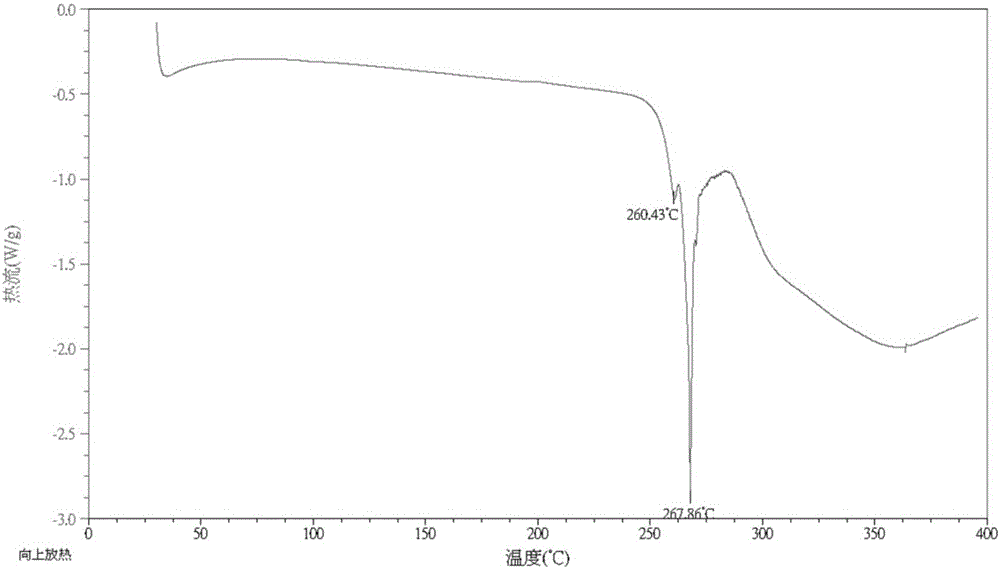
[0066] 1.0 g of the starting material (S)-5-amino-4,11-diethyl-4-hydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b] Quinoline-3,14(4H,12H)dione was dissolved in 100ml of a mixed solvent of tetrahydrofuran and methanol with a volume ratio of 1:1, added 100mL of n-heptane, stirred and precipitated, filtered and dried to obtain a solid product with a yield of 71 %. The code is HSSYO-001-3-THF-MeOH-Ht. Its DSC such as figure 2 As shown, it shows that Form A has endothermic peaks at 260.43°C and 267.86°C. Its XRPD as figure 1 , Figure 5 or its partial enlargement Image 6 As can be seen from the figure, it and the product obtained in Example 1 are both crystal form A.
Embodiment 3
[0068] 1.0 g of the starting material (S)-5-amino-4,11-diethyl-4-hydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b] Quinoline-3,14(4H,12H)dione was dissolved in 100ml of a mixed solvent of tetrahydrofuran and methanol with a volume ratio of 1:3, added 200mL of water, stirred to separate out, filtered and dried to obtain a solid product with a yield of 70% . No. HSSYO-001-3-THF-MeOH-H 2 O, its XRPD as Figure 5 or its partial enlargement Image 6 As can be seen from the figure, it and the products obtained in Example 1 or Example 2 are both crystal form A.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com