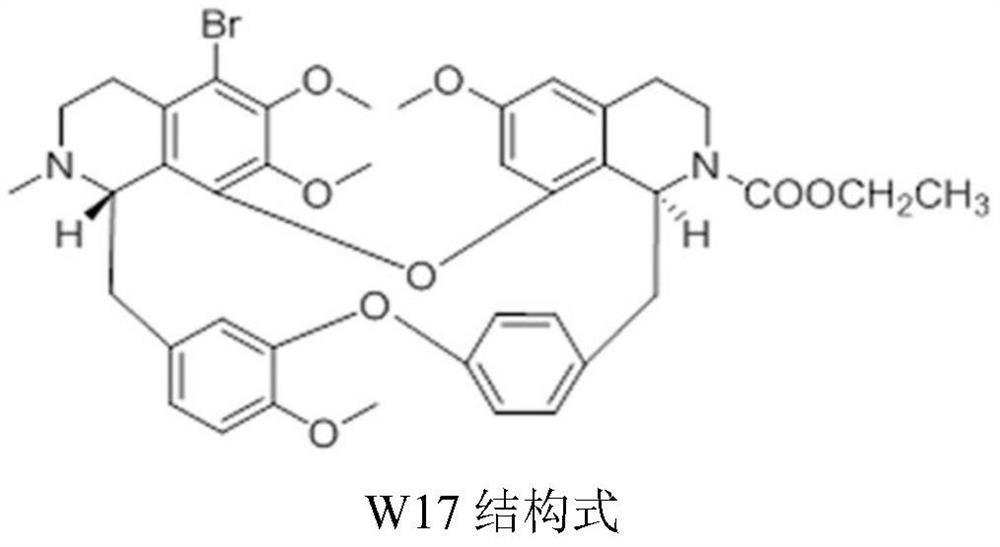
A kind of crystal form of ethyl 5-bromotetrandrine formate and its preparation method
A technology of tetrandrine and ethyl formate, which is applied in the field of medicine and can solve problems such as research on the crystal form of undeveloped compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
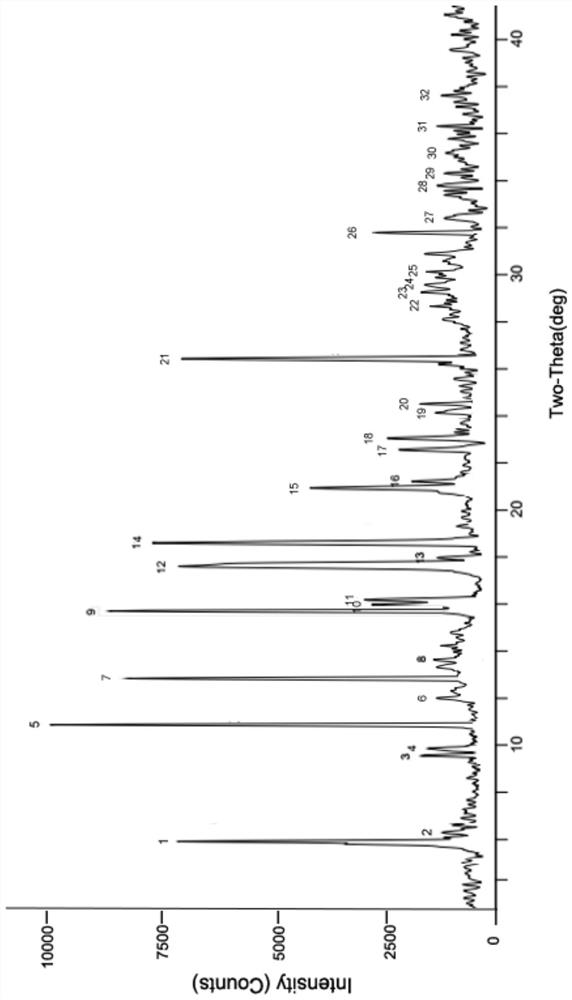
[0046] Weigh 500 mg of 5-bromotetrandrine ethyl formate amorphous (obtained with reference to the literature Ping He et al., JAsianNatProd Res, 2012, 14(6):564-576, the same below), and add it to a 25ml round bottom In the flask, 10 ml of ethanol aqueous solution (7.5 ml of ethanol: 2.5 ml of water) was added. Heat to 70-75°C, stir for 3 hours, add 5ml of acetone dropwise, stir for 30min, then cool down to -5-5°C, crystallize, filter, wash the filter cake with 2ml of acetone, and dry at 50-60°C for 4 hours in vacuum. 423 mg of ethyl 5-bromotetrandrine formate crystal form I was obtained, with a yield of 84.6%. At room temperature, take 50 mg of crystal form I sample for x-ray powder diffraction (RINT 2100Ultima X-ray diffractometer, Cu target, graphite crystal monochromator filter, working voltage: 40kV, current: 150mA, receiving slit: 0.3nm, 2θ Angle is 3°~40°, step size: 0.02°) test, obtained XRPD data of crystal form I, the results are shown in figure 1 .
Embodiment 2
[0048] Weigh 500 mg of amorphous ethyl 5-bromotetrandrine formate, add it into a 25 ml round bottom flask, and add 10 ml of ethanol aqueous solution (8 ml of ethanol: 2 ml of water). Heat to 70-75°C, stir for 3.5 hours, add 4ml of acetone dropwise, stir for 30min, then cool down to -5-0°C, crystallize, filter, wash the filter cake with 2ml of acetone, and dry at 50-60°C for 4 hours in vacuum. 430 mg of ethyl 5-bromotetrandrine formate, crystalline form I, was obtained with a yield of 86.0%.
Embodiment 3
[0050] Weigh 500 mg of amorphous ethyl 5-bromotetrandrine formate, add it into a 25 ml round bottom flask, and add 10 ml of isopropanol aqueous solution (8 ml of isopropanol: 2 ml of water). Heat to 70-75°C, stir for 3.5 hours, add 5ml of acetone dropwise, stir for 30min, then cool down to -5-0°C, crystallize, filter, wash the filter cake with 2ml of acetone, and dry in vacuum at 50-60°C for 4 hours. 418 mg of ethyl 5-bromotetrandrine formate crystal form I was obtained with a yield of 83.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com