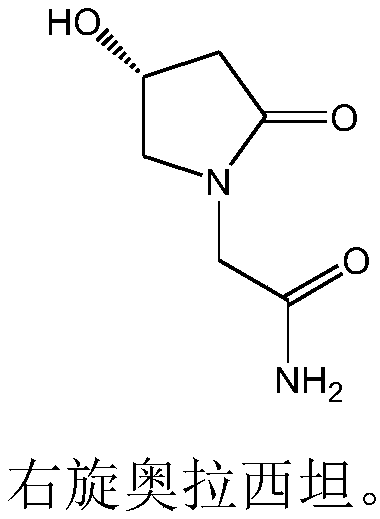
Good-stability right-handed oxiracetam capsule and preparation method thereof
A technology of capsules and pregelatinized starch, which is applied in capsule delivery, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc., and can solve the problem of no further prompts from Dexoxiracetam , to achieve high bioavailability, high dissolution rate and low content of related substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
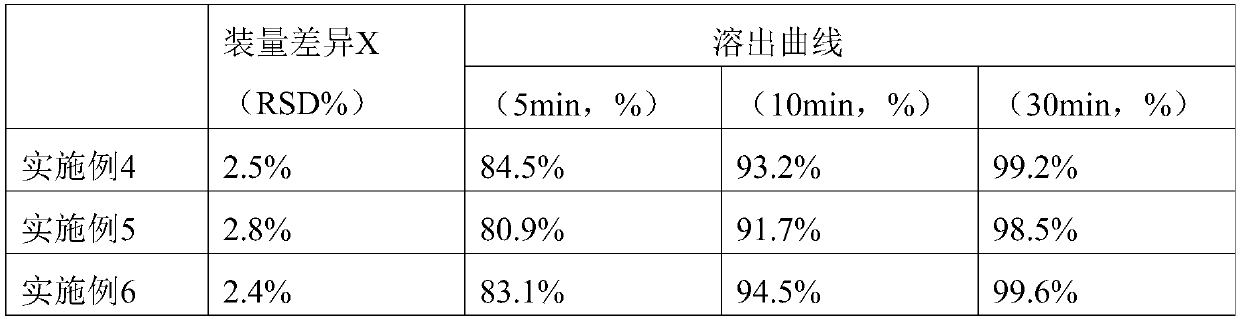
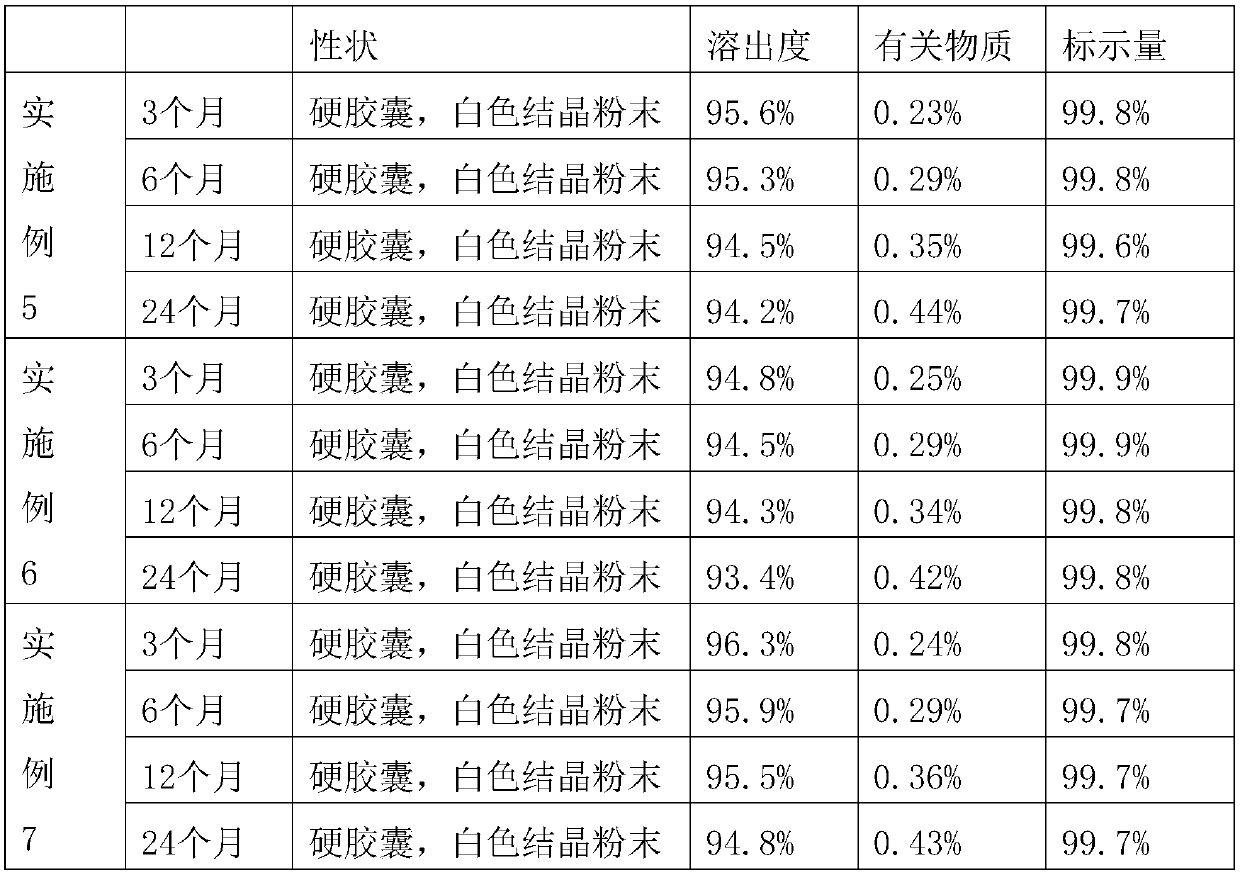
Embodiment 1
[0018] Dissolve 1g of Dexoxiracetam in 6mL of mixed solvent (3mL of dimethylacetamide, 3mL of ethyl acetate), heat to dissolve at 50°C, filter, seal the filtrate, and heat at 180-200r / min Stir at a high speed for about 18 hours, and filter again. After filtration, the filtrate is left to stand in a desiccator to evaporate the solvent to form crystals, collect the crystals, and dry the collected crystals at 30±2°C and a relative humidity of 80±2% for 5 hours to obtain Dexo-oxiracetam crystals. The obtained dextro-oxiracetam crystal is carried out powder diffraction experiment: powder diffraction determination (XRPD): test instrument condition: use Bruker D2PHASER powder diffractometer to carry out normal temperature test, test condition is: with Cu Ka It is the light source, the voltage is 30kV, the current is 10mA, the test step is 0.014°, the scanning speed is 0.1s / step, and the scanning range is 5-40° (2θ). After testing, the D-oxiracetam crystal prepared in Example 1 has ...
Embodiment 2
[0020] Dissolve 2g of Dexoxiracetam in 8mL of a mixed solvent (2mL of dimethylacetamide, 6mL of ethyl acetate), heat to dissolve at 40°C, filter, seal the filtrate, and heat at 150-180r / min Stir at a high speed for about 15 hours, and filter again. After filtration, the filtrate is left to stand in a desiccator to evaporate the solvent to form crystals, collect the crystals, and dry the collected crystals at 20±2°C and a relative humidity of 70±2% for 4 hours to obtain Dexo-oxiracetam crystals. As determined by powder diffraction, the crystalline form of dexoxiracetam prepared in Example 2 is the same as the crystalline form of dexoxiracetam prepared in Example 1.
Embodiment 3
[0022] Dissolve 1g of Dexoxiracetam in 8mL of mixed solvent (3mL of dimethylacetamide, 5mL of ethyl acetate), heat to dissolve at 45°C, filter, cover and seal the filtrate at 220-250r / min Stir at a high speed for about 24 hours, and filter again. After filtration, the filtrate is left to stand in a desiccator to evaporate the solvent to form crystals, collect the crystals, and dry the collected crystals at 40±2°C and a relative humidity of 85±2% for 6 hours to obtain Dexo-oxiracetam crystals. As determined by powder diffraction, the crystalline form of dexoxiracetam prepared in Example 3 is the same as the crystalline form of dexoxiracetam prepared in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com