A kind of crystal form of azilsartan intermediate and preparation method thereof
A kind of technology of intermediate and crystal form, applied in the field of medicine, can solve the problems such as the crystal form and crystal preparation method of intermediate compound I of Azisartan are not found
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Preparation method one of the crystal form of Azilsartan intermediate compound I is as follows:
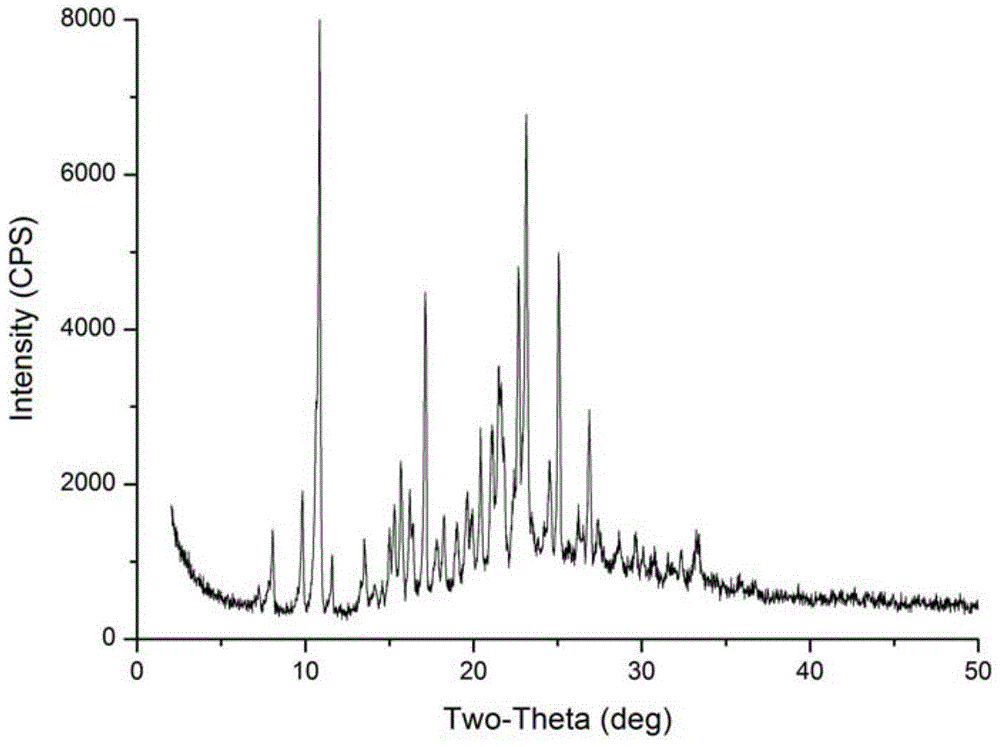
[0026] In a 100mL round-bottom flask, add 5g Azisartan intermediate compound I, add 60mL absolute ethanol, stir, heat to reflux to dissolve all, after refluxing for 1 hour, stop heating, and cool to room temperature (25℃, the same below ), stirring and crystallization at room temperature for 1 hour, filtering, washing with absolute ethanol, and drying to obtain 4.3 g of a white solid sample with a purity of 99.63% and a melting point of 187-189°C.
[0027] The above white solid was subjected to X-ray powder diffraction, and the data obtained are as follows:
[0028] Table 1 Crystal Form X-ray Powder Diffraction Data Table in Example 1
[0029]
[0030]
[0031] However, in the white solid prepared in this example, no regular crystals that can be tested for X-ray single crystal diffraction were not obtained.
Embodiment 2
[0033] Preparation method 2 of the crystalline form of Azilsartan intermediate compound I is as follows:
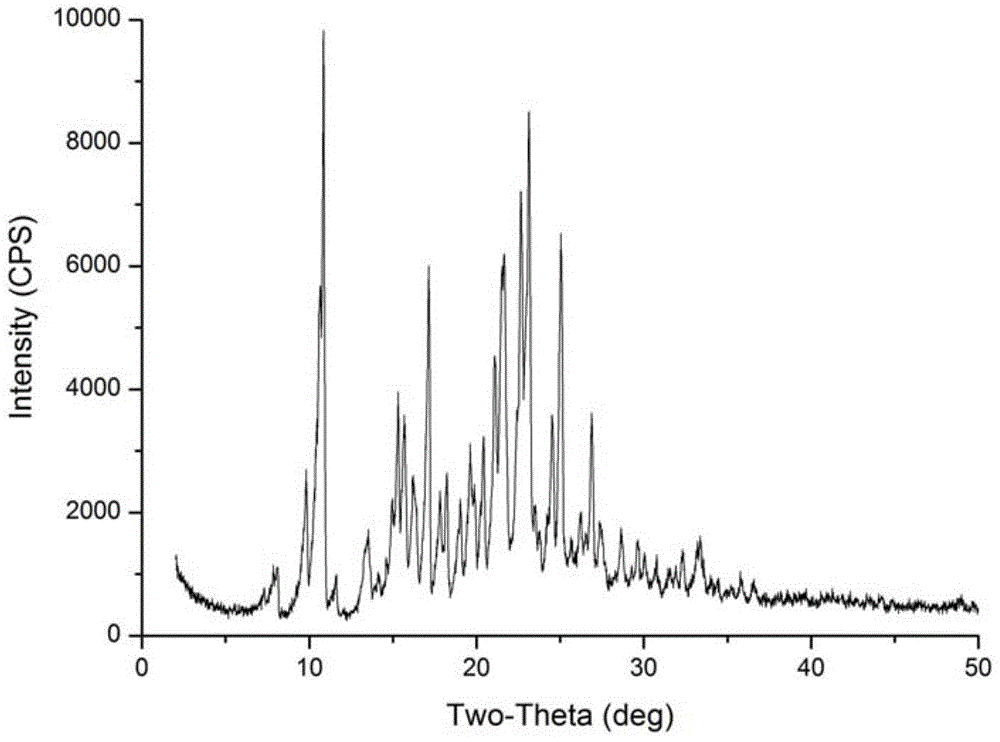
[0034] In a 100mL round-bottom flask, add 5g Azisartan intermediate compound I, add 100mL ethyl acetate, stir, heat to reflux to dissolve all, after refluxing for 1 hour, stop heating, naturally cool to room temperature, stir at room temperature to crystallize After 1 hour, it was filtered, washed with ethyl acetate, and dried to obtain 4.4 g of a white solid sample of a new crystal form with a purity of 99.54% and a melting point of 186-188°C.
[0035] The above white solid was subjected to X-ray powder diffraction, and the data obtained are as follows:
[0036] Table 2 Crystalline X-ray powder diffraction data table in Example 2
[0037]
[0038]
[0039] However, in the white solid prepared in this example, no regular crystals that can be tested for X-ray single crystal diffraction were not obtained.
Embodiment 3
[0041] The method for cultivating the crystals of Azilsartan intermediate compound I is as follows:
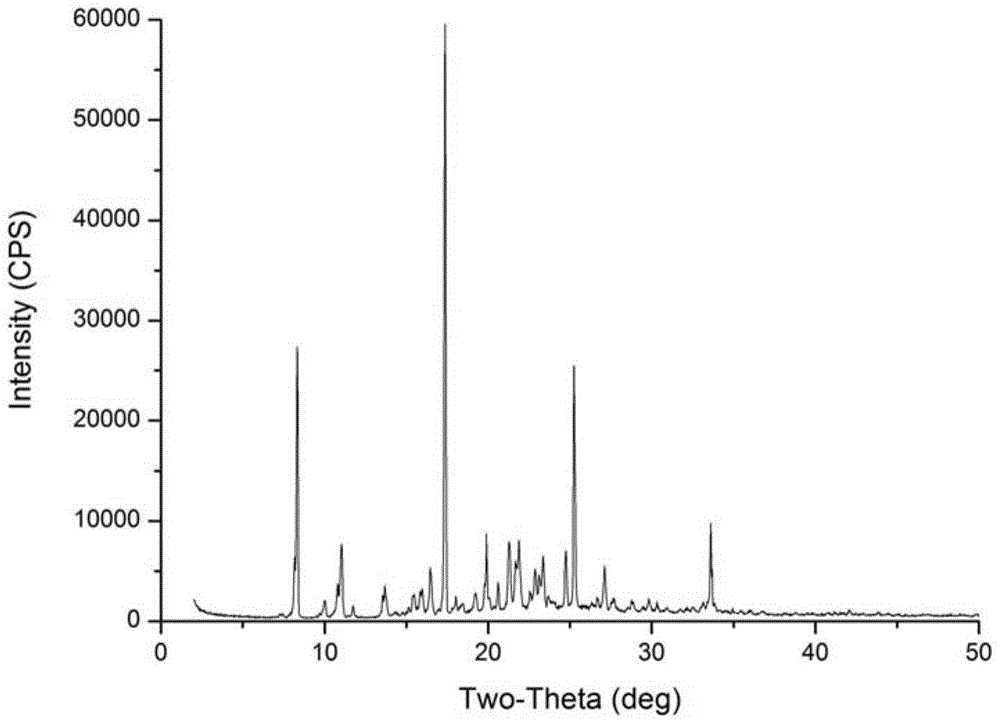
[0042] In a 50mL beaker, add 50mg of Azisartan intermediate compound I, add 10mL of ethyl acetate, heat to dissolve all Azisartan intermediate compound I, stop heating, cool to room temperature, and then add ethyl acetate to 10 mL , Seal the flask with plastic wrap, leave a small hole to slowly permeate the solvent, place it in a stable environment, and let the crystal grow naturally at 20℃. After 3 days, there will be colorless massive crystals. After the crystal growth is complete , Filtered, washed with ethyl acetate, and dried to obtain 43.5 mg of a new crystal sample with a purity of 99.77% and a melting point of 188-189°C.
[0043] After grinding the above crystals into powder, perform X-ray powder diffraction, and the data obtained are as follows:
[0044] Table 3 X-ray powder diffraction data table of the crystal in Example 3
[0045]
[0046]
[0047] Among the crystals prep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com