Preparation method of edoxaban intermediate
A technology for edoxaban and intermediates, which is applied in the field of preparation of edoxaban intermediates, can solve the problem of high production risk of explosive dangerous reagent sodium azide, low diastereomer selectivity, and conversion steps. Cumbersome and other problems, to achieve the effect of reducing production risks, reducing costs, and simplifying operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
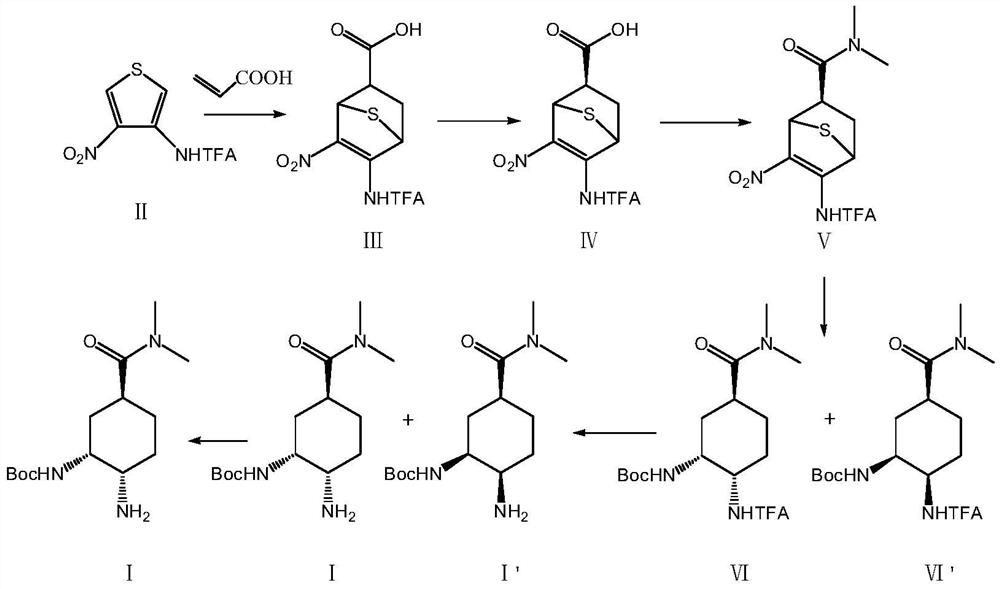
[0043] A preparation method of an edoxaban intermediate, specifically comprising the following reaction steps:
[0044] S1: Add 100.0g (1.00eq.) of compound II thiophene derivative, 31.5g (1.05eq.) of acrylic acid and 500mL (5.0vol.) of diethylene glycol dimethyl ether into a 1L four-necked flask, and raise the temperature to 135°C. Insulate the reaction, and stop the reaction after detecting that the content of the raw compound II is ≤1.0% (6h). Cool down to 5°C, keep warm for 1h, crystallize, filter the system, wash and filter with 50mL (0.5vol.) of pre-cooled diethylene glycol dimethyl ether at 5°C, and dry the filter cake at 50°C to obtain 119.6g Compound III, the yield is 92%, the purity (HPLC) is 99.0%, 1 HNMR (MeOD) 3.98 (d, J = 12.2 Hz, 1H), 3.60 (t, J = 12.4 Hz, 1H), 2.80 (m, 1H), 2.11 (m, 2H).
[0045]The above compound III was subjected to chiral resolution, 500mL (5.0vol.) methanol was added to the reaction flask, the temperature was raised to 25°C, stirring was ...
Embodiment 2
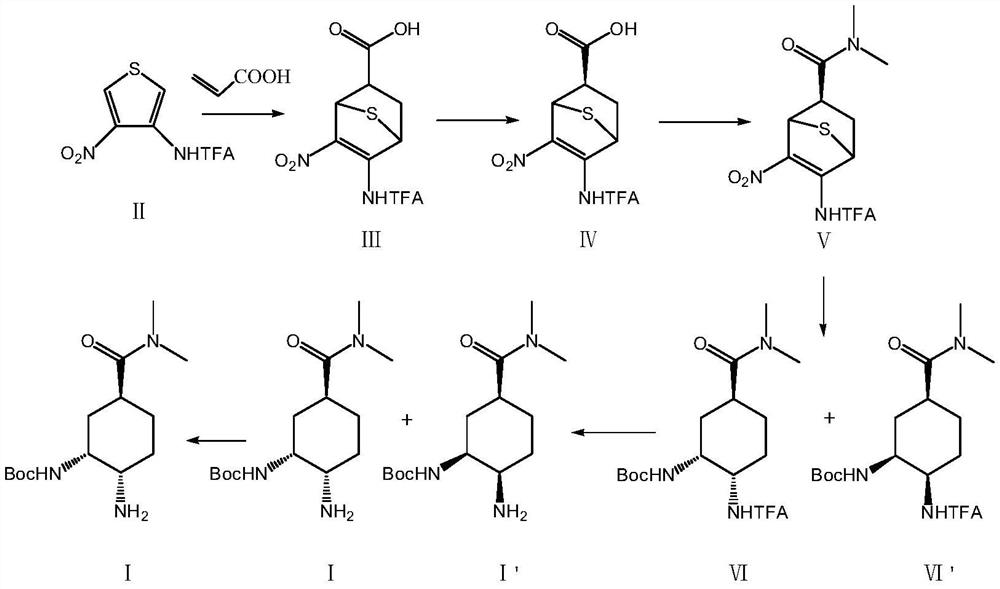
[0053] A preparation method of an edoxaban intermediate, specifically comprising the following reaction steps:
[0054] S1: Add 100.0g (1.00eq.) of compound II thiophene derivative, 32.4g (1.08eq.) of acrylic acid and 500mL (5.0vol.) of diethylene glycol dimethyl ether into a 1L four-necked flask, and raise the temperature to 130°C. Insulate the reaction, and stop the reaction after detecting that the content of the raw compound II is ≤1.0% (8h). Cool down to 0°C, keep warm for 1h, crystallize, filter the system, wash and filter with 50mL (0.5vol.) of diethylene glycol dimethyl ether pre-cooled at 0°C, and dry the filter cake at 45°C to obtain 122.2g Compound III, the yield is 94%, the purity (HPLC) is 99.3%, 1 HNMR (MeOD) 3.98 (d, J = 12.2 Hz, 1H), 3.60 (t, J = 12.4 Hz, 1H), 2.80 (m, 1H), 2.11 (m, 2H).
[0055] The above compound III was subjected to chiral resolution, 500mL (5.0vol.) methanol was added to the reaction flask, the temperature was raised to 20°C, stirring was...
Embodiment 3
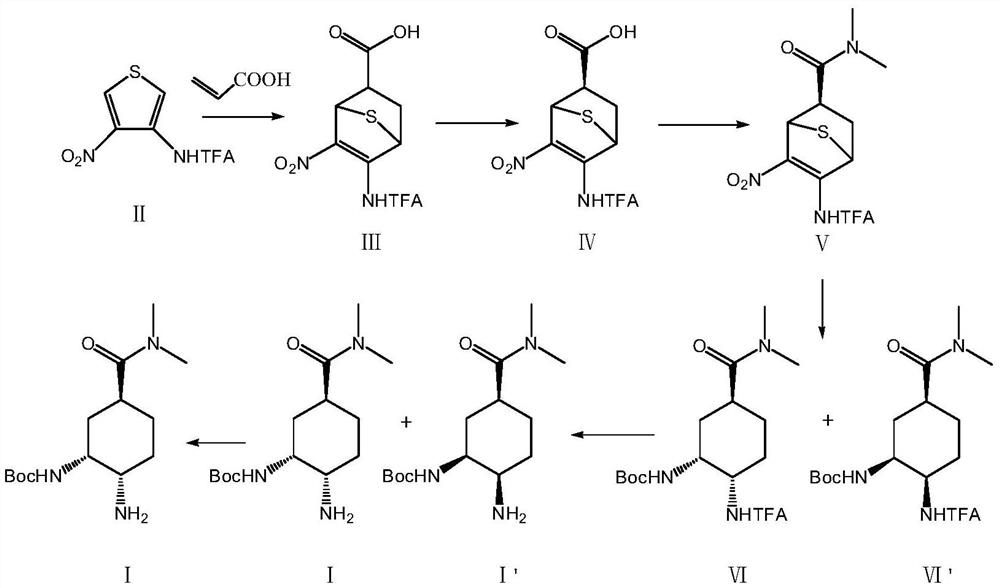
[0063] A preparation method of an edoxaban intermediate, specifically comprising the following reaction steps:
[0064] S1: Add 100.0g (1.00eq.) of compound II thiophene derivative, 33.0g (1.10eq.) of acrylic acid and 500mL (5.0vol.) of diethylene glycol dimethyl ether into a 1L four-necked flask, and raise the temperature to 140°C. Insulate the reaction, and stop the reaction after detecting that the content of the raw compound II is ≤1.0% (4h). Cool down to 10°C, keep warm for 1h, crystallize, filter the system, wash and filter with 50mL (0.5vol.) of diethylene glycol dimethyl ether pre-cooled at 10°C, and dry the filter cake at 55°C to obtain 120.9g Compound III, the yield is 93%, the purity (HPLC) is 99.1%, 1 HNMR (MeOD) 3.98 (d, J = 12.2 Hz, 1H), 3.60 (t, J = 12.4 Hz, 1H), 2.80 (m, 1H), 2.11 (m, 2H).
[0065] The above compound III was subjected to chiral resolution, 500mL (5.0vol.) methanol was added to the reaction flask, the temperature was raised to 30°C, stirring w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com