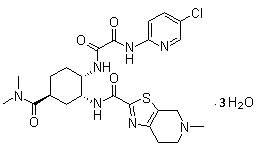
Edoxaban trihydrate as well as preparation method and application thereof
A technology of shaban trihydrate and edoxaban, which is applied in the field of medicine, can solve the problems of high total amount of impurities, large number of edoxaban impurities, and difficulty in repeating the crystal form
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
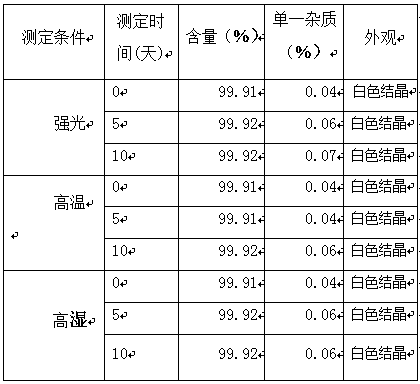
[0031] In a 100L reaction kettle, add 6 kg of edoxaban (purity: 98.06%, HPLC) and 65 L of acetone-acetonitrile-ammonia water = 9:1:0.5 mixture, heat to 50°C-55°C, and keep warm for 20 minutes. Strain while hot. The filtrate was kept at 35°C-38°C for 1.5 hours; then naturally cooled to room temperature and held for 2.5 hours, the precipitated crystals were filtered and dried naturally at room temperature to obtain 5.71 kg of white crystals with a purity of 99.91%. The solvent residue test met the requirements. The moisture measured by Karl Fischer method is 8.97%.
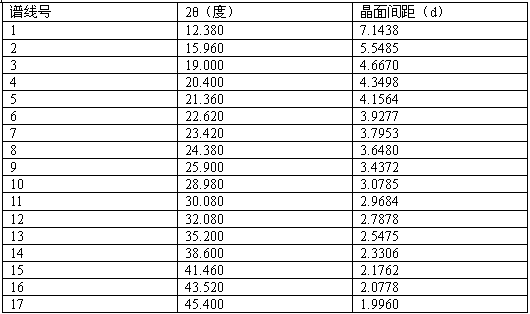
[0032] Instrument model and measurement conditions: Rigaku D / max 2500 diffractometer; CuKa 40Kv 100mA; 2θ scanning range: 0-50 ° .
Embodiment 2
[0034] In a 100L reaction kettle, add 6 kg of edoxaban (purity 98.06%, HPLC) and 60 L of acetone-acetonitrile-ammonia water = 9:0.8:0.3 mixture, heat to 50°C-53°C, and keep warm for 20 minutes , filtered while hot. The filtrate was kept at 35°C-36°C for 1 hour; then naturally cooled to room temperature and held for 2 hours, the precipitated crystals were filtered and dried naturally at room temperature to obtain 5.56 kg of white crystals with a purity of 99.92%. The solvent residue test met the requirements. The moisture measured by Karl Fischer method is 8.98%.
[0035] The X-ray diffraction result that records is with embodiment 1.
Embodiment 3
[0037] In a 100L reaction kettle, add 6 kg of edoxaban (purity 98.06%, HPLC) and 70 L of acetone-acetonitrile-ammonia water = 9:0.5:0.7 mixture, heat to 52°C-54°C, and keep warm for 20 minutes , filtered while hot. The filtrate was kept at 37°C-38°C for 2 hours; then naturally cooled to room temperature and kept for 3 hours, the precipitated crystals were filtered and dried naturally at room temperature to obtain 5.66 kg of white crystals with a purity of 99.92%, and the solvent residue test met the requirements. The moisture measured by Karl Fischer method is 8.88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com