Intermediate for preparing edoxaban free alkali, and preparation method and application of intermediate
A technology of edoxaban and free base, applied in the preparation of intermediates of edoxaban free base and its preparation field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089]
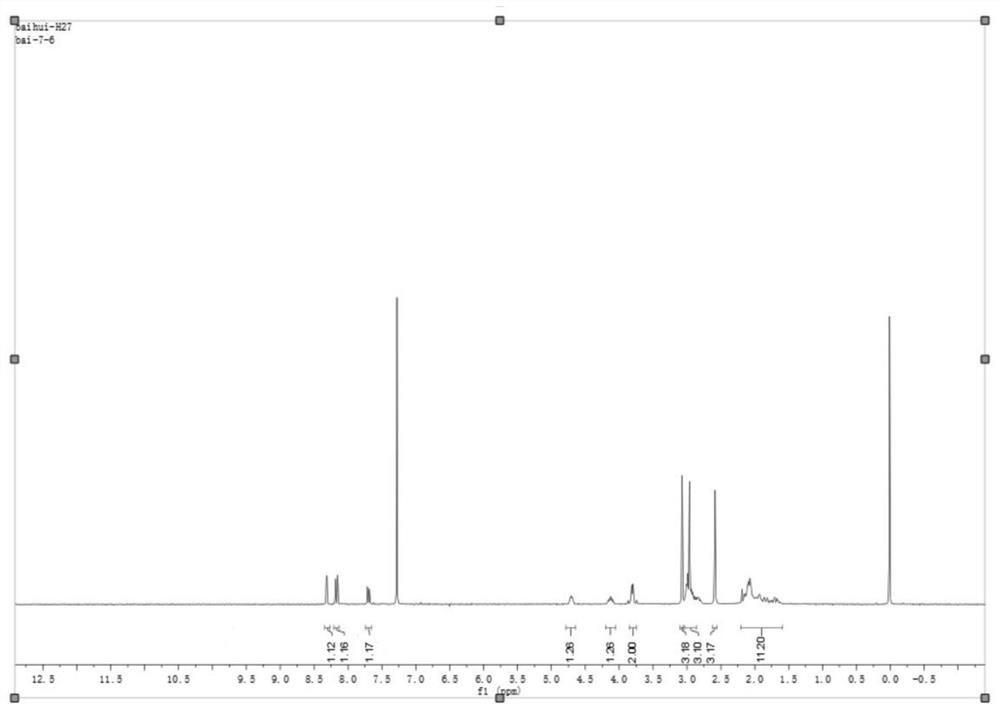
[0090] Under the protection of nitrogen, put 23.47g (0.1mol) 5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-into a 500ml three-necked flask Formate hydrochloride, 250ml dichloromethane, 20ml N,N-dimethylacetamide, 2ml 4-picoline. Adjust the temperature to -10 to -15°C, and slowly add 20.24g (0.2mol) triethylamine dropwise. Control the temperature from -10 to -15°C, and add 12.06g (0.1mol) of pivaloyl chloride dropwise. After the dripping is completed, the temperature is controlled at -10 to -15°C and stirred for 2 hours to obtain solution A, which is kept at -10 to -15°C for later use. Solution A needs to be freshly prepared and stored at low temperature.
[0091]
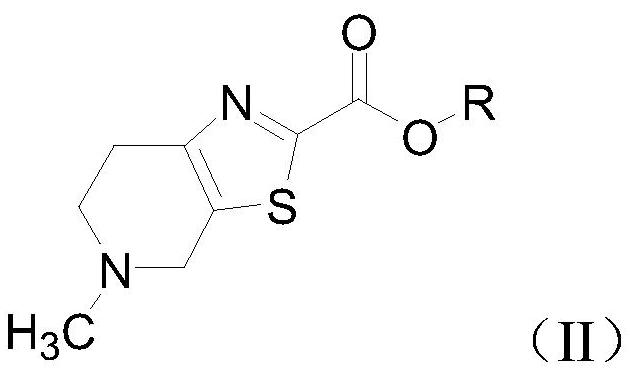
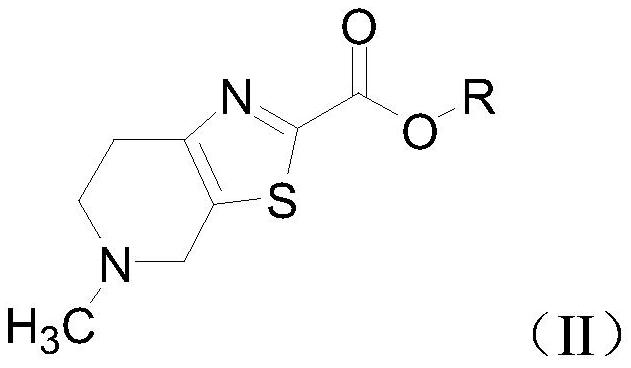
[0092] Among them, R is a trimethylacetyl group.
[0093] In another 1000ml reaction flask, add 56g (0.1mol) of methanesulfonate of compound (IV) and 250ml of dichloromethane. The temperature was lowered to -10 to -15°C, and 20.24g (0.2mol) of triethylamine was added dropwise. Control the temper...
Embodiment 2
[0097]
[0098] Under the protection of nitrogen, put 23.47g (0.1mol) 5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-into a 500ml three-necked flask Formate hydrochloride, 250ml dichloromethane, 20ml N,N-dimethylacetamide, 2ml 4-picoline. Adjust the temperature to -35 to -40°C, and slowly add 20.24g (0.2mol) triethylamine dropwise. Control the temperature from -35 to -40°C, and add 12.06g (0.1mol) of pivaloyl chloride dropwise. After the dropping is completed, the temperature is controlled at -35 to -40°C and the reaction is stirred for 2 hours to obtain solution A, which is kept at -35 to -40°C for later use. Solution A needs to be freshly prepared and stored at low temperature.
[0099]
[0100] Among them, R is trimethylacetyl.
[0101] In another 1000ml reaction flask, add 56g (0.1mol) of methanesulfonate of compound (IV) and 250ml of dichloromethane. The temperature was lowered to -35 to -40°C, and 20.24g (0.2mol) of triethylamine was added dropwise. Control th...
Embodiment 3
[0104]
[0105] Under the protection of nitrogen, put 23.47g (0.1mol) 5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-into a 500ml three-necked flask Formate hydrochloride, 250ml dichloromethane, 20ml N,N-dimethylacetamide, 2ml 4-picoline. Adjust the temperature to 5-10°C, and slowly add 20.24g (0.2mol) triethylamine dropwise. The temperature was controlled at 5 to 10°C, and 12.06g (0.1mol) of pivaloyl chloride was added dropwise. After the dripping is completed, the temperature is controlled at 5 to 10°C and the reaction is stirred for 2 hours to obtain solution A, which is kept at 5 to 10°C for later use. Solution A needs to be newly prepared for immediate use and stored at low temperature.
[0106]
[0107] Among them, R is trimethylacetyl.
[0108] In another 1000ml reaction flask, add 56g (0.1mol) of methanesulfonate of compound (IV) and 250ml of dichloromethane. The temperature was lowered to 5-10°C, and 20.24g (0.2mol) of triethylamine was added dropwise. Cont...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com