Synthesis method of edoxaban
A compound and tertiary amine technology, applied in the field of compound preparation, can solve the problems of large solvent consumption, cumbersome post-processing, unsuitable for industrial production, etc., and achieve the effects of improving yield and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
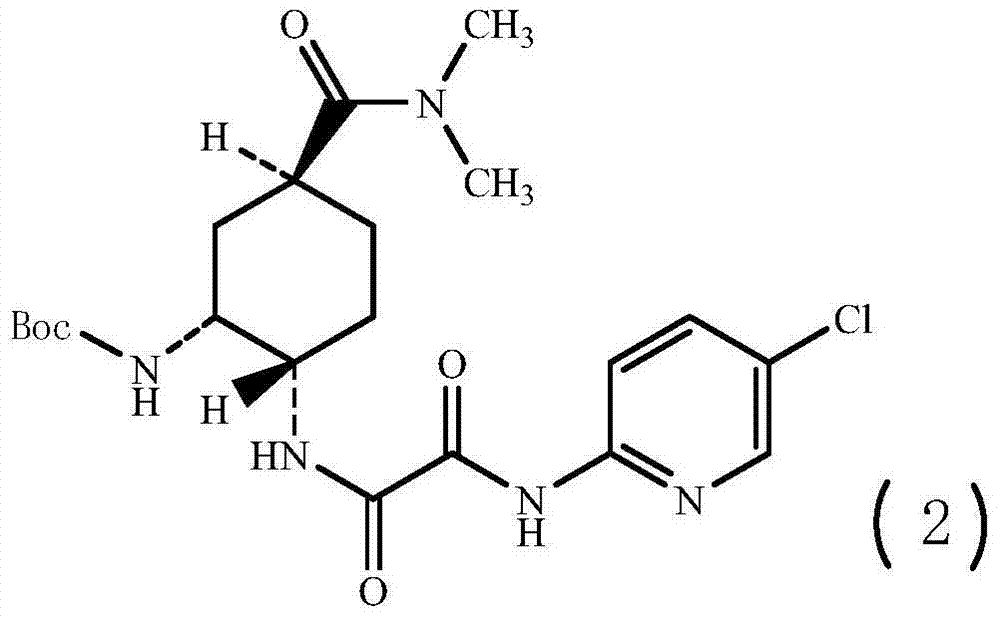
[0044] (1R,2S,5S)-2-({2-[5-Chloropyridin-2-yl)amino]-2-oxoacetyl}amino)-5-[(dimethylamino)carbonyl]cyclohexyl - tert-butyl carbamate, preparation of compound (2)
[0045]
[0046] where Boc represents tert-butoxycarbonyl
[0047] Add 560mL acetonitrile, 560.0g (1S,2R,4S)-1-amino-4-(dimethylaminocarbonyl)-cyclohexyl-2-carbamic acid tert-butyl oxalate to a 1000mL reaction flask, stir, 25 Add 64.9g triethylamine at ±2°C, stir for 10min, add 47.5g ethyl 2-[(5-chloropyridine)amino]-2-oxoacetate monohydrochloride, heat up to 60°C, and react at 60±2°C 6h, lower the temperature to 25°C, react at 25±2°C for 16h, no raw material point detected by TLC. Developing solvent: dichloromethane: methanol = 10:1.
[0048] After the reaction is complete, add pure water to crystallize, cool to 10°C, keep warm at 10±2°C for crystallization for 1 hour, filter with suction, wash the filter cake with water, and air-dry at 50°C for 10 hours to obtain 51.4 g of white solid.
Embodiment 2
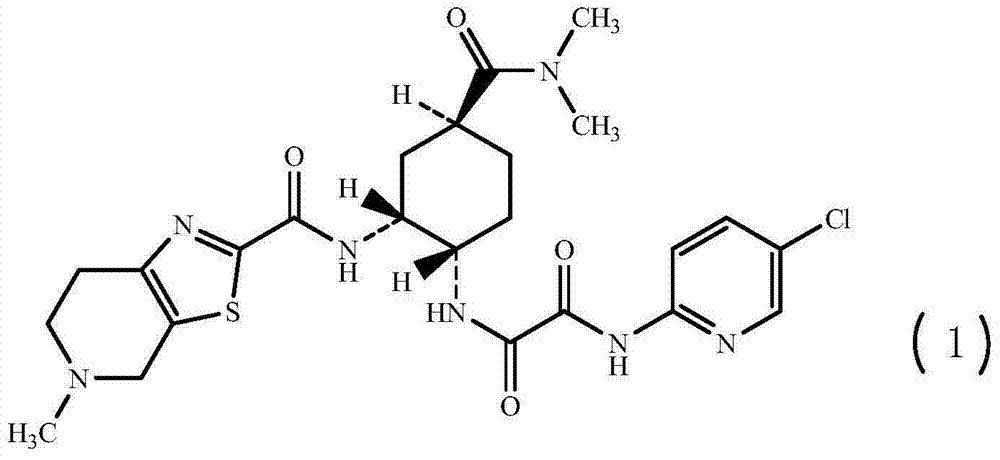
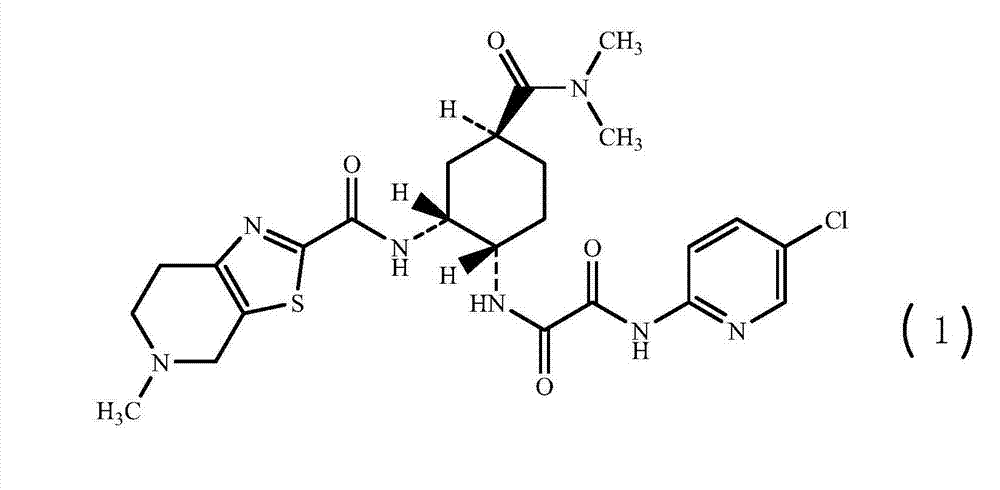
[0050] N-(5-chloropyridin-2-yl)-N'-((1S,2R,4S)-4-[(dimethylamino)carbonyl]-2-{[(5-methyl-4,5 , 6,7-tetrahydrothiazolo[5,4-c]pyridin-2-yl)carbonyl]amino}cyclohexyl)oxalamide, the preparation of compound (1)
[0051]
[0052] Add 2560mL acetonitrile, 128.0g (1R,2S,5S)-2-({2-[5-chloropyridin-2-yl)amino]-2-oxoacetyl}amino)-5- [(Dimethylamino)carbonyl]cyclohexyl-tert-butyl carbamate, stir, add 131.4g methanesulfonic acid at 25±2°C, keep the reaction for 2h, TLC detects that there is no raw material point, cool down, add 152.2g three at 10°C Ethylamine, stirred for 10min, then added 70.6g 5-methyl-4,5,6,7-tetrahydrothiazolo[5,4-c]pyridine-2-carboxylate hydrochloride, 40.7g 1-hydroxybenzene Triazole and 62.9g of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride were heated up to 25°C and reacted at 25±2°C for 18h. TLC detects that there is no raw material point. Developing solvent: dichloromethane: methanol = 10:1.
[0053] After the reaction, cool down to 10°C, add ...
Embodiment 3
[0063] N-(5-chloropyridin-2-yl)-N'-((1S,2R,4S)-4-[(dimethylamino)carbonyl]-2-{[(5-methyl-4,5 , 6,7-tetrahydrothiazolo[5,4-c]pyridin-2-yl)carbonyl]amino}cyclohexyl)oxalamide p-toluenesulfonic acid monohydrate, the preparation of compound (1-a)
[0064]
[0065] 220mL dichloromethane, 20.9g N-(5-chloropyridin-2-yl)-N'-((1S,2R,4S)-4-[(dimethylamino)carbonyl]- 2-{[(5-methyl-4,5,6,7-tetrahydrothiazolo[5,4-c]pyridin-2-yl)carbonyl]amino}cyclohexyl)oxalamide, stirred to make all the solids Dissolve, add 38.2ml 1mol / L p-toluenesulfonic acid ethanol solution at 25±2°C, react for 1h, after the reaction is completed, remove the solvent under reduced pressure, add 313.5mL 85% ethanol aqueous solution to the residue, and keep warm at 60±2°C After 1 hour, the temperature was lowered to 10°C for suction filtration, and vacuum-dried to constant weight at 25°C to obtain 23.0 g of a white solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com