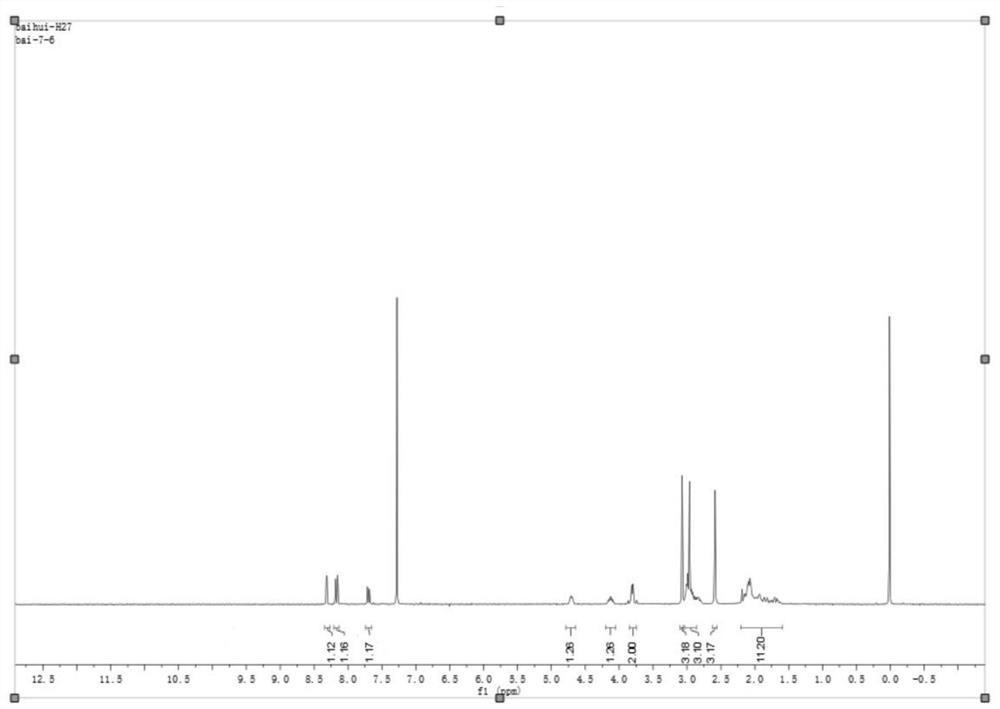
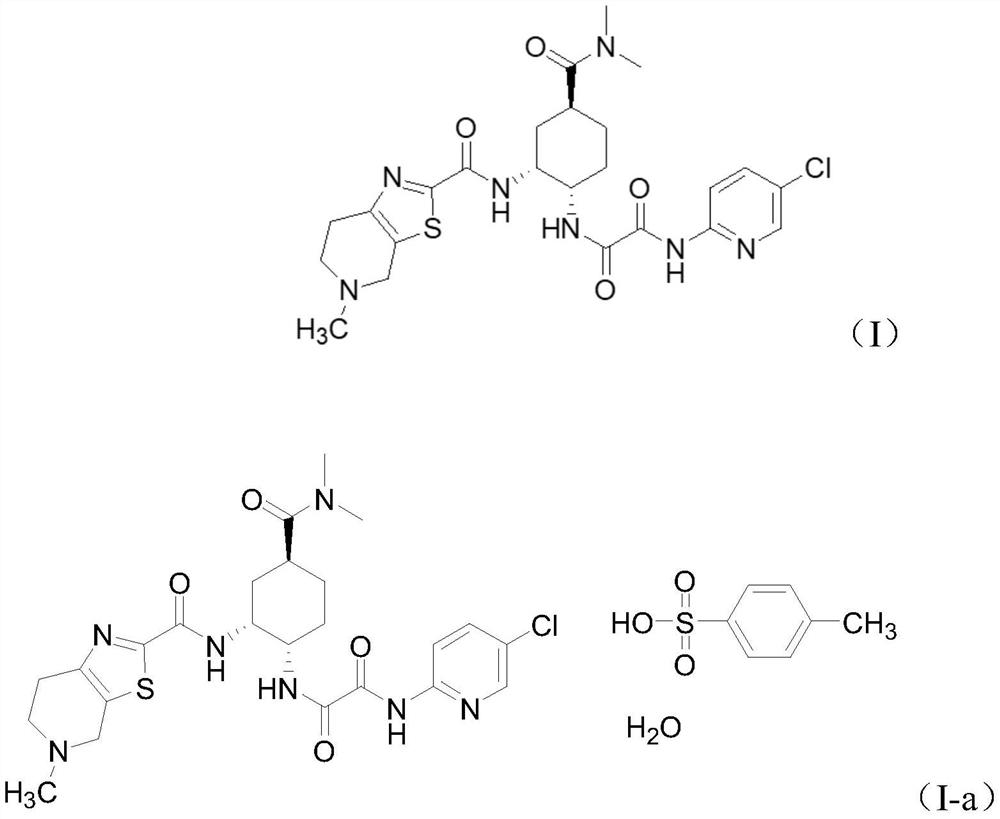
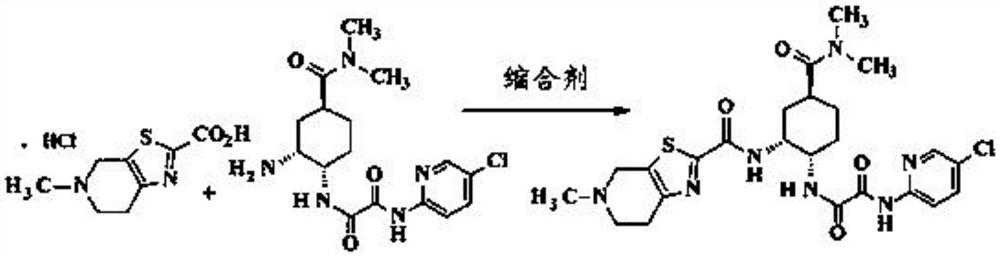
Intermediate for the preparation of edoxaban free base and its preparation method and application
A technology for edoxaban and free base is applied in the preparation of intermediates for edoxaban free base and the field of preparation thereof, and achieves the effects of simple production process, low production cost and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089]
[0090] Under nitrogen protection, 23.47g (0.1mol) of 5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2- Formic hydrochloride, 250ml dichloromethane, 20ml N,N-dimethylacetamide, 2ml 4-picoline. Adjust the temperature to -10 to -15°C, and slowly add 20.24 g (0.2 mol) of triethylamine dropwise. Control the temperature from -10 to -15°C, and add 12.06 g (0.1 mol) of pivaloyl chloride dropwise. After the dropwise addition, the temperature was controlled at -10 to -15°C and the reaction was stirred for 2 hours to obtain solution A, which was kept at -10 to -15°C for later use. Solution A needs to be freshly prepared immediately before use and stored at low temperature.
[0091]
[0092] Wherein, R is a trimethylacetyl group.
[0093] In another 1000ml reaction flask, 56g (0.1mol) of methanesulfonate of compound (IV) and 250ml of dichloromethane were added. The temperature was lowered to -10 to -15°C, and 20.24 g (0.2 mol) of triethylamine was added dropwis...
Embodiment 2
[0097]
[0098] Under nitrogen protection, 23.47g (0.1mol) of 5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2- Formic hydrochloride, 250ml dichloromethane, 20ml N,N-dimethylacetamide, 2ml 4-picoline. Adjust the temperature to -35 to -40°C, and slowly add 20.24 g (0.2 mol) of triethylamine dropwise. Control the temperature from -35 to -40°C, and add 12.06 g (0.1 mol) of pivaloyl chloride dropwise. After the dropwise addition is completed, the temperature is controlled at -35 to -40°C and the reaction is stirred for 2 hours to obtain solution A, which is kept at -35 to -40°C for later use. Solution A needs to be freshly prepared immediately before use and stored at low temperature.
[0099]
[0100] Wherein, R is a trimethylacetyl group.
[0101] In another 1000ml reaction flask, 56g (0.1mol) of methanesulfonate of compound (IV) and 250ml of dichloromethane were added. The temperature was lowered to -35 to -40°C, and 20.24 g (0.2 mol) of triethylamine was add...
Embodiment 3
[0104]
[0105] Under nitrogen protection, 23.47g (0.1mol) of 5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2- Formic hydrochloride, 250ml dichloromethane, 20ml N,N-dimethylacetamide, 2ml 4-picoline. Adjust the temperature to 5 to 10° C., and slowly add 20.24 g (0.2 mol) of triethylamine dropwise. Control the temperature from 5 to 10°C, and add 12.06 g (0.1 mol) of pivaloyl chloride dropwise. After the dropwise addition is completed, the temperature is controlled at 5 to 10°C and the reaction is stirred for 2 hours to obtain solution A, which is kept at 5 to 10°C for later use. Solution A needs to be freshly prepared immediately before use and stored at low temperature.
[0106]
[0107] Wherein, R is a trimethylacetyl group.
[0108] In another 1000ml reaction flask, 56g (0.1mol) of methanesulfonate of compound (IV) and 250ml of dichloromethane were added. The temperature was lowered to 5 to 10°C, and 20.24 g (0.2 mol) of triethylamine was added dropwise. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com