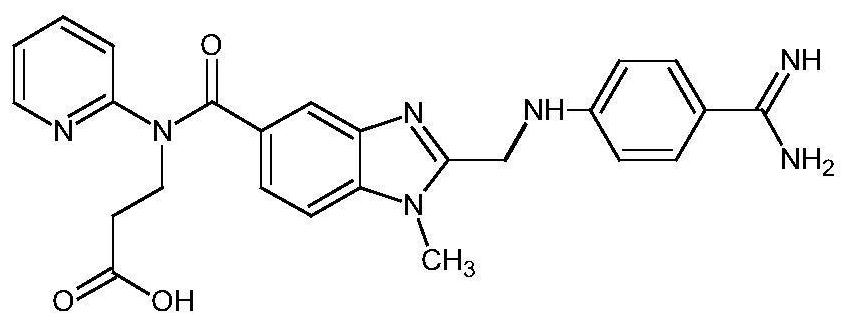
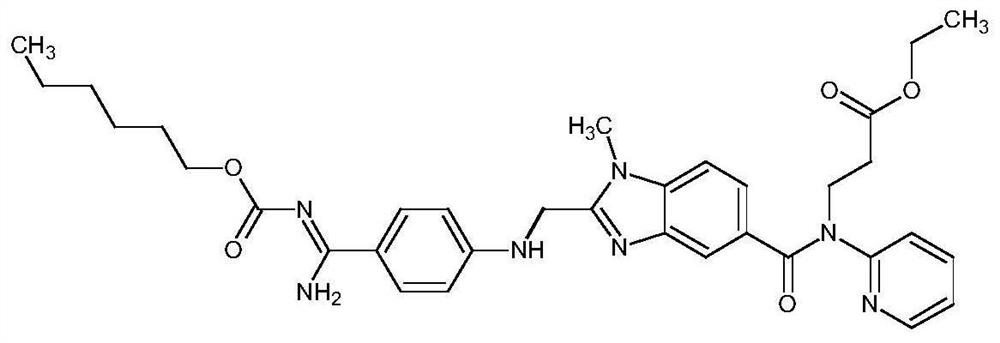
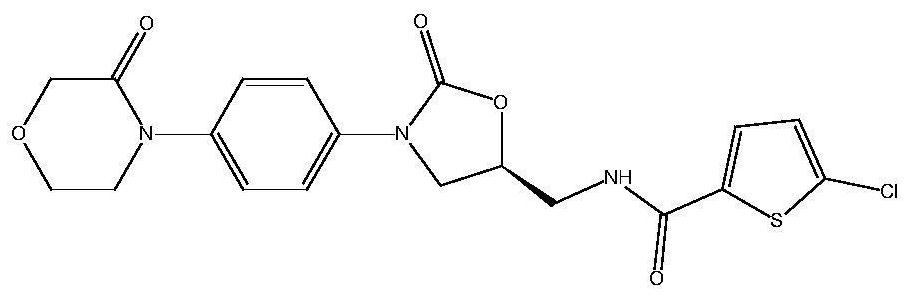
Method for simultaneously determining concentrations of anticoagulant drugs and active metabolite in plasma
A metabolite, plasma technology, applied in the field of plasma drug concentration detection, can solve the problems of low sensitivity and simultaneous detection of dabigatran etexilate without public disclosure, and achieve the effect of high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] This embodiment provides the preparation method of reference substance solution, mixed internal standard working solution, standard curve and quality control plasma samples:
[0057] 1. Preparation of reference solution:
[0058] Accurately weigh appropriate amounts of dabigatran etexilate, dabigatran, rivaroxaban, edoxaban, and apixaban reference substances, dissolve them in methanol and constant volume to obtain a concentration of 1 mg·mL -1 Dabigatran etexilate, dabigatran, edoxaban and apixaban stock solutions, and a concentration of 0.75mg·mL -1 The rivaroxaban stock solution, as shown in Table 1, was stored in a -20°C refrigerator. Dabigatran etexilate, dabigatran, rivaroxaban, apixaban and edoxaban stock solution are prepared to contain 5000ng / mL dabigatran etexilate, dabigatran, ritoxaban Reference solution of varoxaban, apixaban and edoxaban.
[0059] Table 1: Stock Solution Preparation
[0060]
[0061]
[0062] 2. Preparation of rivaroxaban-deuteriu...
Embodiment 2
[0075] This example provides the detection of anticoagulant drugs dabigatran etexilate, rivaroxaban, edoxaban, apixaban and active metabolites in pretreated plasma by ultra-high performance liquid chromatography tandem mass spectrometry The method for dabigatran specifically comprises the following steps:
[0076] Step 1. Pretreatment of plasma samples and standard curve plasma samples
[0077] Plasma sample preparation:
[0078] Take 100 μL of plasma sample in a 1.5 mL centrifuge tube, add 890 μL of ice-cold methanol and 1 μg·mL -1 Mix 10 μL of the internal standard solution, vortex and centrifuge (13000g, 4°C, 10min), take the supernatant, blow it to dryness with nitrogen, add 100μL methanol solution containing 1% formic acid to the dry sample and ultrasonically reconstitute, centrifuge (13000g, 4°C, 10 min), take 50 μL of the supernatant, add 450 μL of pure water, mix well, and wait for sample injection analysis.
[0079] Standard curve plasma sample preparation:
[008...
Embodiment 3
[0098] Using the plasma samples provided by the subjects taking anticoagulant drugs, the method in Example 2 was used to analyze the concentration of anticoagulant drugs, and the results were as follows Figure 3.1-3.3As shown in , the determination of the presence of rivaroxaban in one case of plasma samples and the presence of dabigatran etexilate and its metabolite dabigatran in the other case of plasma samples further confirmed the existence of rivaroxaban, dabigatran etexilate and their metabolites The concentration of the product dabigatran was 263.7ng L -1 , 4.4ng·L -1 and 224.0ng·L -1 .
[0099] 【Test verification】
[0100] 1. Linearity
[0101] The weighted linear regression was performed on the concentration (X, ng / mL) of the peak area ratio (Y) of the drug to the internal standard. Among them, the weight coefficient is 1 / X, and the correlation coefficient r of the regression equation is greater than 0.997, as shown in Table 6. The linearity is good and meets th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com