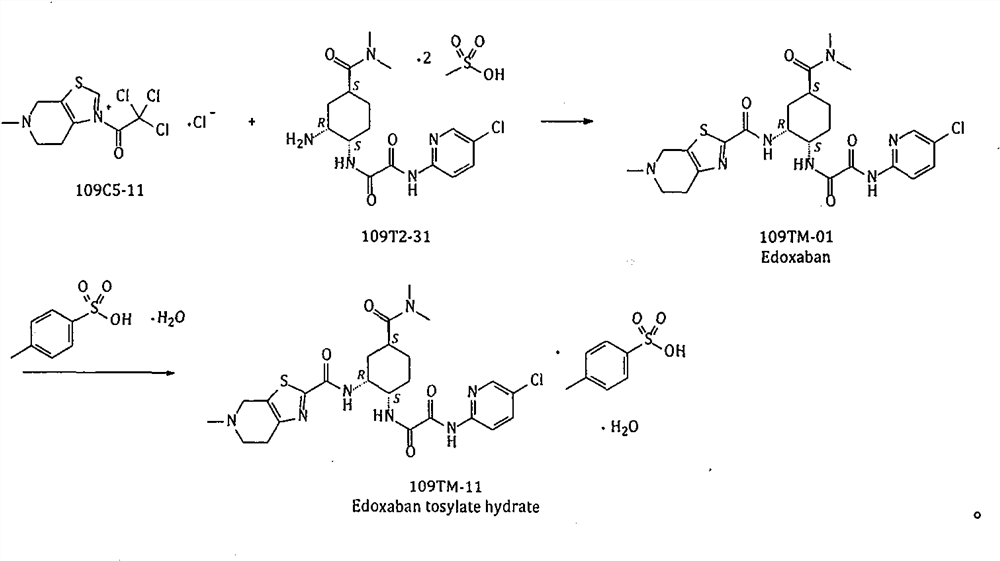
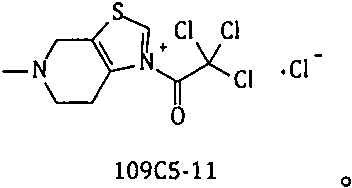
A kind of method for preparing edoxaban from trichloroethyl ketonium salt derivative
A technology of edoxaban and ethyl ketone, which is applied in the field of medicinal chemistry, can solve the problems of high cost, many production safety hazards, and unsuitability for industrial-scale production, and achieve the effect of saving dosage and simplifying post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
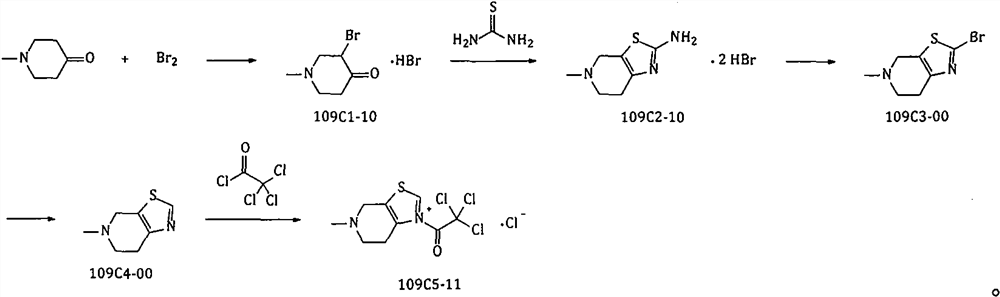
[0037] Example 1 Synthesis of 3-bromo-1-methyl-4-piperidone hydrobromide
[0038]
[0039] In the reactor, add glacial acetic acid (500kg), temperature control is not more than 25 ℃, drip 1-methyl-4-piperidone (100kg, 883.6mol), then drip 48% aqueous hydrobromic acid (150kg, ~ 892.5mol); temperature control is not more than 20 ℃, drip liquid bromine (141.6kg, 885.8mol), after dripping, stir at room temperature overnight; shake off filtration, collect solid, dry; Obtain about 237kg of 10C1-10 dry product (theoretical Quantity: 241.2kg). Yield: 98.3%.
Embodiment 2
[0040] Example 2 Synthesis of 4,5,6,7-tetrahydro-5-methylthiazolo[5,4-c]pyridin-2-amine dihydrobromide
[0041]
[0042] In the reaction kettle, add dehydrated alcohol (900kg), add thiourea (72kg, 945.9mol), add the 109C1-10 (235kg, 860.9mol) gained in Example 1, reflux reaction for about 36~48hr; , cooled and crystallized; filtered, rinsed with absolute ethanol, collected the solid; dried to obtain about 242kg (theoretical amount: 285.0kg) of 109C2-10 dry product. Yield: 84.9%.
Embodiment 3
[0043] Example 3 Synthesis of 2-bromo-4,5,6,7-tetrahydro-5-methylthiazolo[5,4-c]pyridine
[0044]
[0045] In the reactor, add drinking water (1200kg), add 48% aqueous hydrobromic acid solution (600kg, ~ 3559mol), add the 240kg (724.9mol) 109C2-10 gained in Example 2, after stirring, the temperature control is not more than 10 ℃, dropwise add the solution prepared by sodium nitrite 75kg (1087mol) and drinking water (360kg), after the dropwise addition, the reaction is stirred at room temperature for 3hr. After the reaction was completed, the temperature was controlled not to exceed 20°C, and the pH of the system was adjusted to ≥13 with 30% liquid caustic soda; extracted three times with toluene; the organic phases were combined, dried with anhydrous sodium sulfate, filtered, and concentrated in toluene under reduced pressure to obtain a brown oily residue 109C3-00 is about 123 kg (the liquid phase shows that it contains about 8.7% of 109C4-00; the theoretical amount calcul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com