Preparation method of edoxaban and intermediate thereof
A technology for edoxaban and its intermediates, which is applied in the field of preparation of edoxaban and its intermediates, and can solve the problems of large-scale production costs, harsh reaction conditions, and low reaction yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0054]
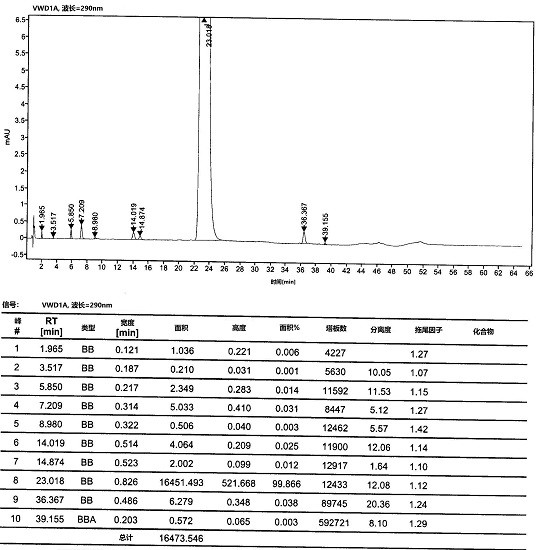
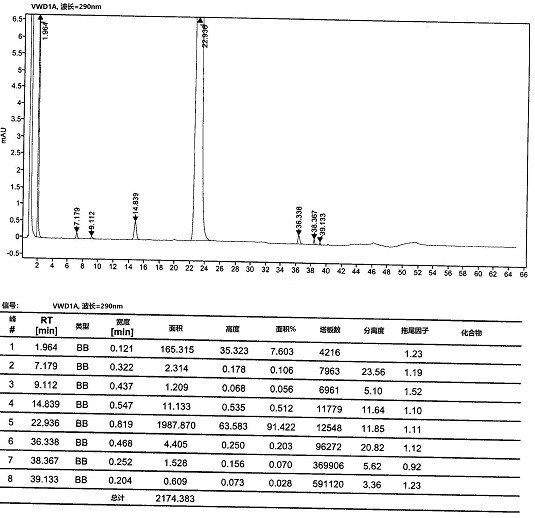
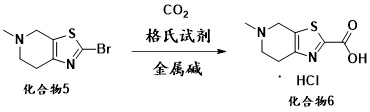
[0055] At room temperature, compound 5 (10 g, 0.043 mol) and 200 mL of tetrahydrofuran were added to a 500 mL three-necked flask, and the system was cooled to 0 °C and the atmosphere was replaced with nitrogen. At low temperature of 0°C, 21 mL (20.58 g, 0.2 mol) of isopropylmagnesium chloride was added dropwise, and after stirring for one hour, 1.2 g of lithium hydroxide was added to the reaction system and carbon dioxide gas was continuously introduced and stirred for 2 to 3 hours. After the reaction was completed, concentrated hydrochloric acid was added dropwise to the reaction system to quench the reaction, and the stirring was continued for 2-3 hours. After the reaction, the reaction solution was filtered, the filter cake was washed and refined with methanol / toluene, and dried at 50°C to obtain compound 6 (9.2 g), with a molar yield of 91% and an HPLC purity of 98.35%.
Embodiment 1-2
[0057] At room temperature, compound 5 (10 g, 0.043 mol) and 200 mL of 2-methyltetrahydrofuran were added to a 500 mL three-necked flask, and the system was cooled to 0 °C and the atmosphere was replaced with nitrogen. At low temperature of 0°C, 21 mL (20.58 g, 0.2 mol) of isopropylmagnesium chloride was added dropwise, and after stirring for one hour, 1.2 g of lithium hydroxide was added to the reaction system and carbon dioxide gas was continuously introduced and stirred for 2 to 3 hours. After the reaction was completed, concentrated hydrochloric acid was added dropwise to the reaction system to quench the reaction, and the stirring was continued for 2-3 hours. After the reaction, the reaction solution was filtered, the filter cake was washed and refined with methanol / toluene, and dried at 50°C to obtain compound 6 (8.9 g), with a molar yield of 88% and an HPLC purity of 97.85%.
Embodiment 1-3
[0059] At room temperature, compound 5 (10 g, 0.043 mol) and 200 mL of tetrahydrofuran were added to a 500 mL three-necked flask, and the system was cooled to 0 °C and the atmosphere was replaced with nitrogen. At a low temperature of 0°C, 33mL of cyclohexylmagnesium chloride (28.74g, 0.2mol) was added dropwise, and after stirring for one hour, 1.2g of lithium hydroxide was added to the reaction system and carbon dioxide gas was continuously introduced and stirred for 2 to 3 hours. After the reaction was completed, concentrated hydrochloric acid was added dropwise to the reaction system to quench the reaction, and the stirring was continued for 2-3 hours. After the reaction, the reaction solution was filtered, the filter cake was washed and refined with methanol / toluene, and dried at 50°C to obtain compound 6 (8.5 g), with a molar yield of 84% and an HPLC purity of 98.14%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com