Method for Separating Edoxaban and Its Isomers
A technology of edoxaban and isomers, which is applied in the field of medicinal chemistry and can solve the problems of inability to separate isomer impurities from each other and separation of two substances from each other.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
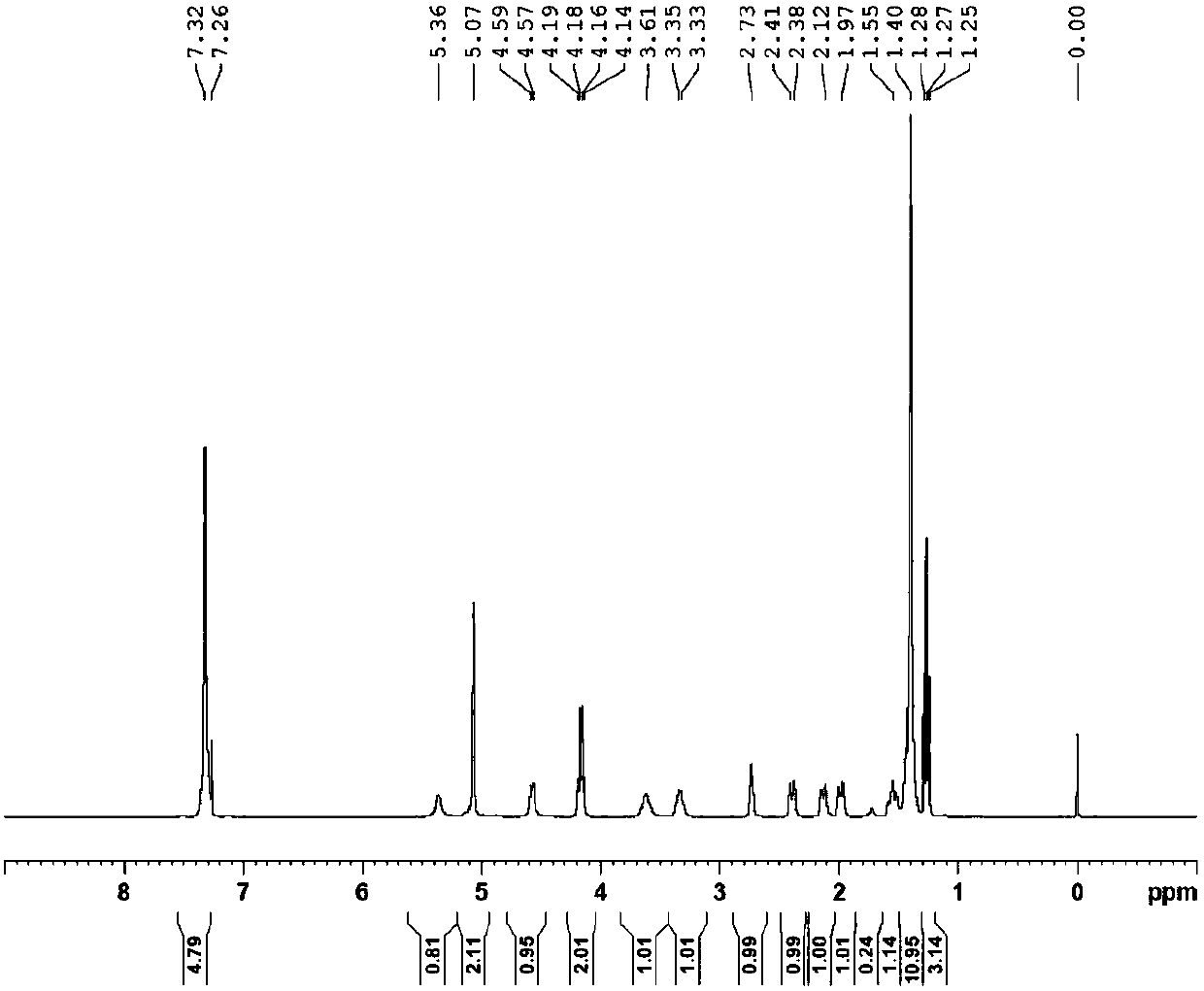
Embodiment 1N
[0051] Example 1N 1 -(5-chloropyridin-2-yl)-N 2 -((1R,2S,4S)-4-[(dimethylamino)carbonyl]-2-{[(5-methyl-4,5,6,7-tetrahydrothiazolo[5,4-c Synthesis of ]pyridin-2-yl)carbonyl]amino}cyclohexyl)ethylenediamine (YDB-RSS)
[0052] 1-1: Synthesis of (1R,4R,5R)-4-iodo-6-oxabicyclo[3.2.1]oct-7-one (YDB-A02)
[0053]
[0054] Add YDB-A001 (236g, 0.954mol) into ethyl acetate (1180ml) and 2M hydrochloric acid (1062ml), stir for 1h, separate the liquids, extract the aqueous phase with ethyl acetate, combine the organic phases, use water for the organic phases, and saturated chlorinate After washing with sodium, drying over anhydrous sodium sulfate, the mother liquor was concentrated to dryness under reduced pressure to obtain 115 g of an oily product (YDB-A01).
[0055] Add YDB-A01 (115g, 0.911mol), potassium iodide (196.7g, 1.185mol), sodium bicarbonate (99.56, 1.185mol) into dichloromethane (1150ml) and water (1140ml), and stir for 10min. Cool down to 10°C, add iodine (300.8g, 1185mo...
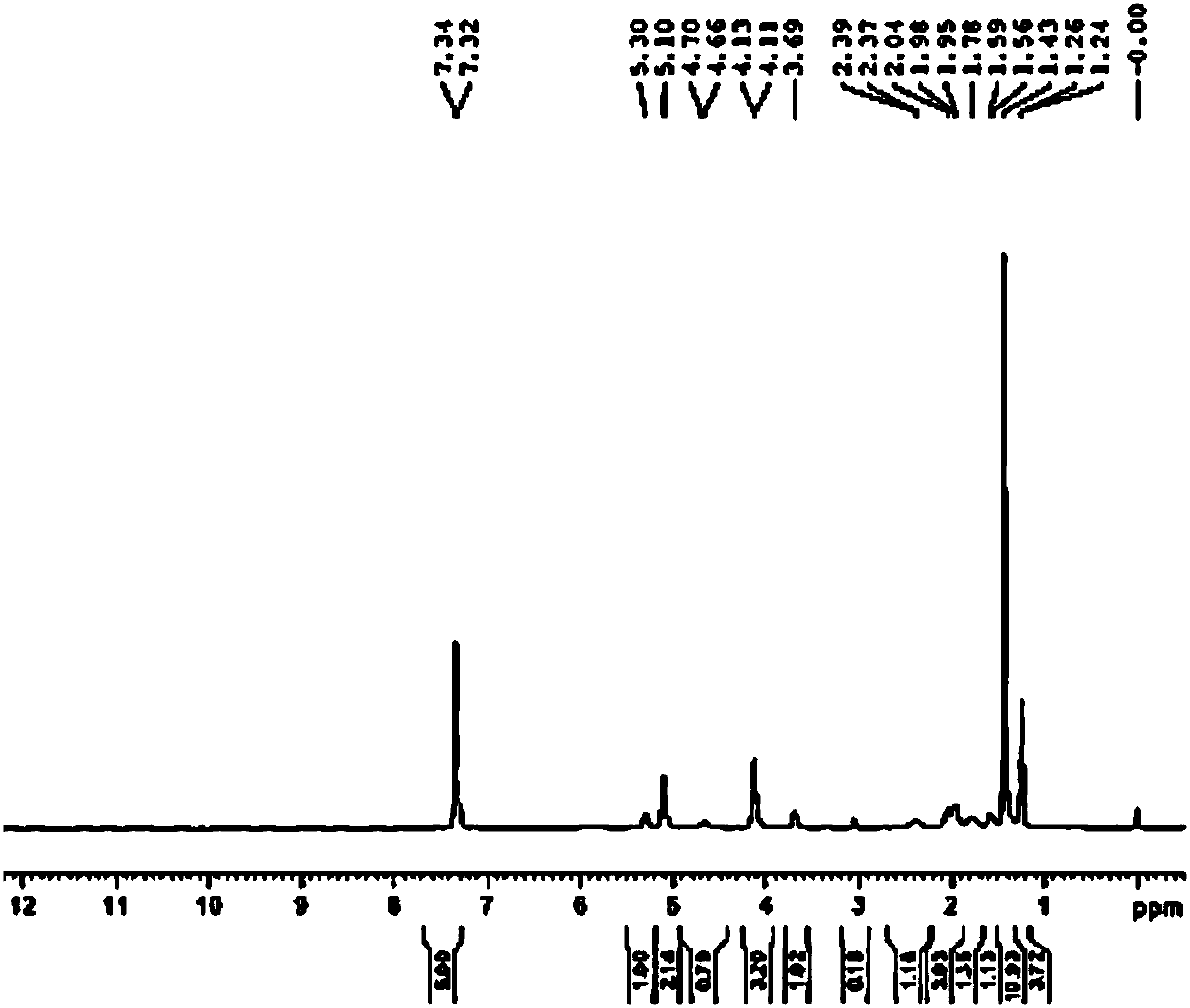
Embodiment 2
[0077] Example 2N 1 -(5-chloropyridin-2-yl)-N 2 -((1R,2S,4R)-4-[(dimethylamino)carbonyl]-2-{[(5-methyl-4,5,6,7-tetrahydrothiazolo[5,4-c Synthesis of ]pyridin-2-yl)carbonyl]amino}cyclohexyl)ethylenediamine (YDB-RSR)
[0078] 2-1:
[0079]
[0080] Dissolve YDB-AC-RSR (11.9g, 0.025mol) in ethanol (600ml), add saturated hydrochloric acid-ethanol solution (130ml), and react at room temperature for 18h. The reaction solution was concentrated under reduced pressure. The product was dissolved in DMF (600ml), and triethylamine (16.4g, 0.163mol), EDCI (5.75g, 0.03mol), HOBT (18.3g, 0.136mol), 5-methyl-4,5,6, 7-Tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-carboxylate hydrochloride (7.04g, 0.03mol), reacted at room temperature for 12h. Add water and dichloromethane, extract, separate liquid, extract the aqueous phase with dichloromethane, combine the organic phases, wash with saturated sodium chloride, dry over anhydrous sodium sulfate, concentrate to dryness under reduced pressure, ...
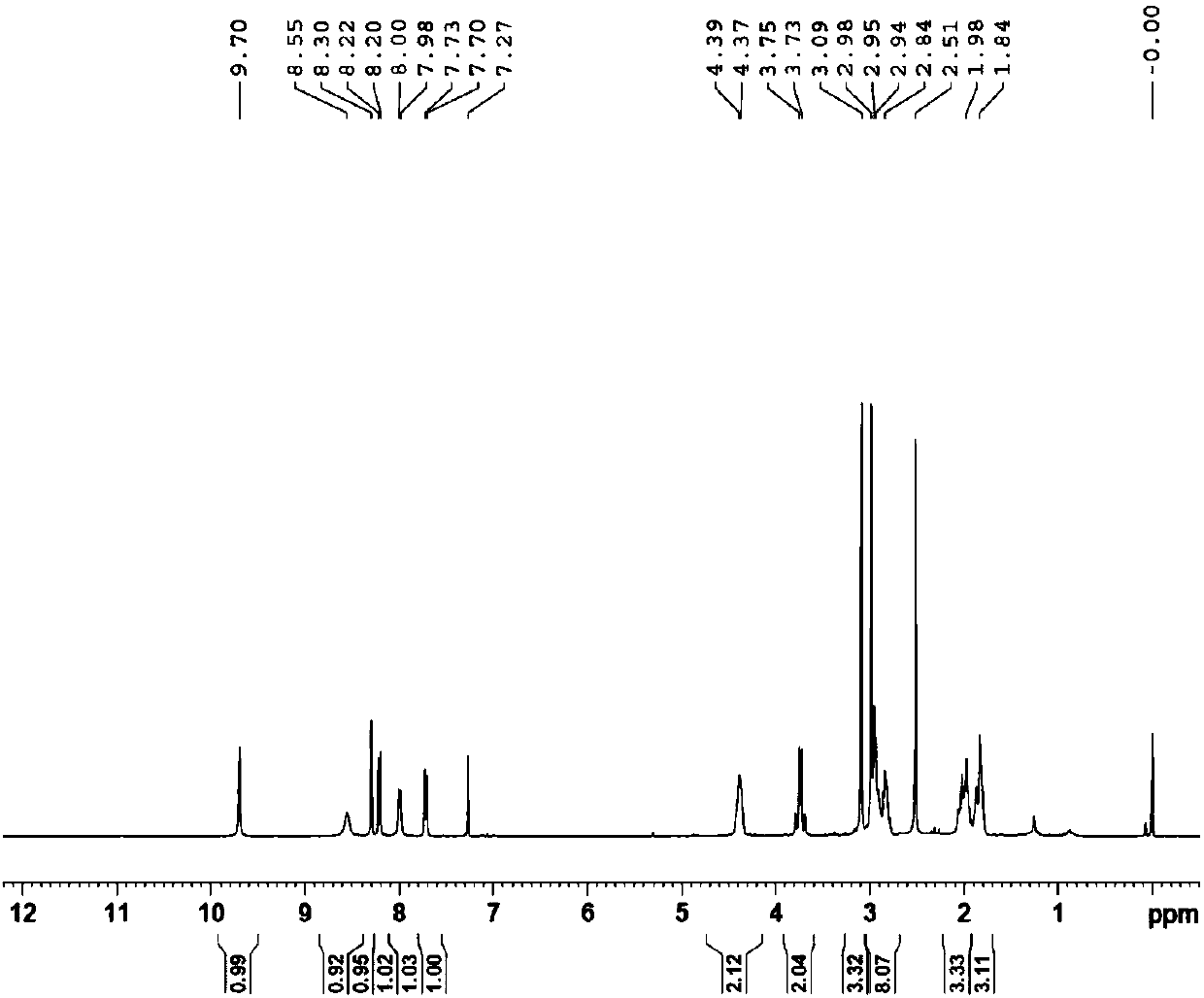
Embodiment 3
[0082] Example 3N 1 -(5-chloropyridin-2-yl)-N 2 -((1S,2S,4S)-4-[(Dimethylamino)carbonyl]-2-{[(5-methyl-4,5,6,7-tetrahydrothiazolo[5,4-c Synthesis of ]pyridin-2-yl)carbonyl]amino}cyclohexyl)ethylenediamine (YDB-SSS)
[0083] 3-1: (1S,2S,4R)-2-[(tert-butoxycarbonyl)amino]-4-[(dimethylamino)carbonyl]cyclohexylcarbamate benzyl ester and (1S,2S,4S Synthesis of )-2-[(tert-butoxycarbonyl)amino]-4-[(dimethylamino)carbonyl]cyclohexylcarbamate benzyl ester (YDB-B11)
[0084]
[0085] Under the protection of argon, dissolve YDB-B08 (22g, 0.052mol) in 220ml ethanol, add dropwise 20% sodium ethoxide solution (24.5ml, 0.063mol), react at 20-30°C for 10h, cool in an ice bath, add 1mol / L Sodium dihydrogen phosphate (440ml), ethyl acetate (500ml), extraction, liquid separation, the aqueous phase was extracted with ethyl acetate, the organic phases were combined, washed with saturated sodium chloride, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com