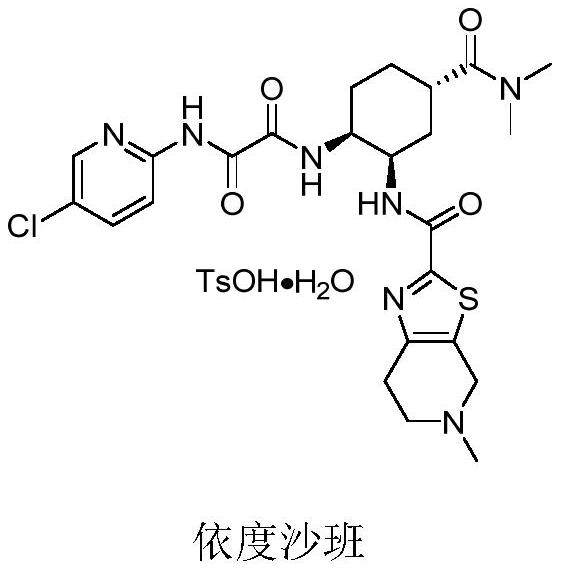
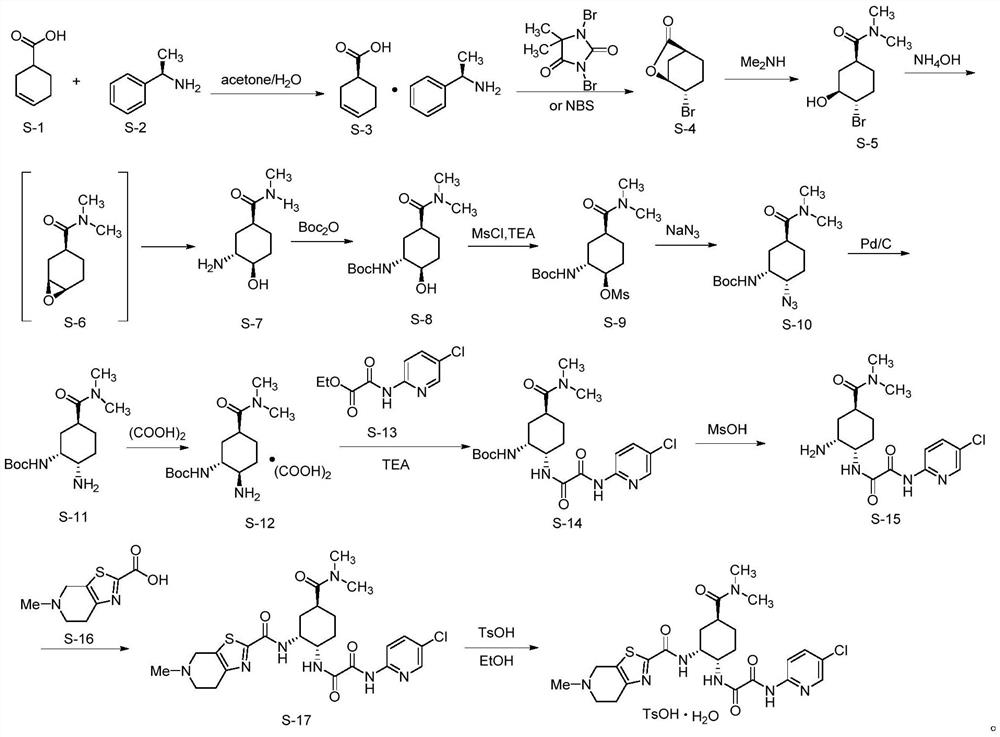
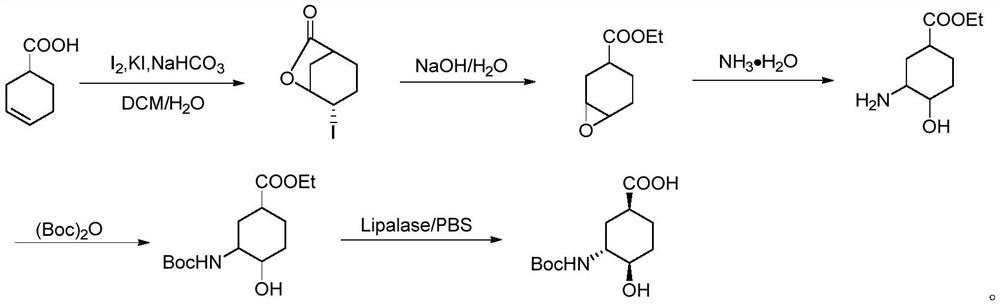
A chiral amino compound, its preparation method and application, and its preparation method for preparing an edoxaban intermediate
A technology of amino compounds and edoxaban, which is applied in the preparation of organic compounds, preparation of carbamic acid derivatives, chemical instruments and methods, etc., can solve the problems of increased production costs and high prices, and achieve low cost, easy operation, The effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] This example provides The synthesis method is as follows:
[0075] Provide a 5mL sealed tube, add nitroene sequentially (1 mmol), catalyst (20mol%), add 2mL of 1,4-dioxane and a stir bar, acetaldehyde (10mmol). The reaction was detected by TLC until the nitroalkene raw material disappeared. Spin to dry, column chromatography (petroleum ether / ethyl acetate=4:1) to get the product. Yield 86%.
[0076] Optical purity values determined by chiral HPLC column, ee: 95%.
[0077] NMR analysis: 1 H NMR (400MHz, CDCl 3 )δ:9.74(s,1H),7.84(dd,J=5.5,3.1Hz,2H),7.74(dd,J=5.5,3.1Hz,2H),5.57-5.44(m,1H),5.07(dd ,J=13.2,9.6Hz,1H),4.77(dd,J=13.2,4.6Hz,1H),3.24(qd,J=18.6,7.2Hz,2H). 13 C NMR (101MHz, CDCl 3 )δ: 197.2, 167.4, 134.6, 131.3, 123.8, 74.5, 43.3, 43.1.
Embodiment 2
[0079] This example provides The specific method is as follows:
[0080] The product of Example 1 was dissolved in tetrahydrofuran as a reactant (1mmol), and 2-diethoxyoxyphosphonic acid ethyl ester (1.5mmol) and sodium carbonate (2mmol) were added at 0°C, and then reacted at 0°C, TLC monitors the reaction until the reaction is complete, the reaction is quenched with saturated ammonium chloride solution, extracted three times with dichloromethane, the organic phase is washed with saturated sodium chloride, dried over anhydrous sodium sulfate, the solvent is evaporated under reduced pressure, column chromatography (eluent: Petroleum ether / ethyl acetate=4:1) to obtain the product. Yield 40%.
[0081] The ratio of diastereoisomers was determined by proton NMR, dr=20:1.
[0082] NMR analysis: 1 H NMR (400MHz, CDCl 3 )δ7.83(dd, J=5.4,3.1Hz,2H),7.73(dd,J=5.4,3.1Hz,2H),6.99-6.93(m,1H),5.65(td,J=11.2,5.7Hz ,1H),4.93(td,J=11.2,6.2Hz,1H),4.23(q,J=7.1Hz,2H),3.31(dd,J=17.1,5.4Hz,1H...
Embodiment 3
[0084] This example provides Synthesis.
[0085] Method 1: Provide 5mL sealed tubes, add nitroolefins sequentially (1 mmol), catalyst (20mol%), add 2mL of methanol and a stir bar, acetaldehyde (10mmol). The reaction was detected by TLC until the nitroenamine raw material disappeared. Spin to dry, column chromatography (petroleum ether / ethyl acetate=4:1) to get the product. Yield 56%.
[0086] Optical purity values determined by chiral HPLC column, ee = 50%.
[0087] NMR analysis: 1 H NMR (400MHz, CDCl 3 )δ:9.74(s,1H),5.20(d,J=7.3Hz,1H),4.74(dd,J=13.6,7.0Hz,1H),4.64-4.52(m,2H),2.93(dd,J =10.3,5.8Hz,2H),1.42(s,9H). 13 C NMR (101MHz, CDCl 3 )δ: 199.0, 154.8, 80.67, 44.9, 44.3, 28.2.
[0088] Method 2:
[0089] Its synthetic route is as follows:
[0090]
[0091] Compound 1 (the product of Example 1) was dissolved in dichloromethane: isopropanol: water (1:6:1 by volume) solution, then added 7 equivalents of sodium borohydride, stirred at room temperature for r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com