Method for separation and determination of edoxaban tosylate hydrate and its isomer impurities by chiral high performance liquid chromatography
A technology of edoxaban tosylate and high performance liquid chromatography, which is applied in the field of drug analysis, can solve problems such as the detection of edoxaban tosylate hydrate isomers that have not yet been seen, and achieve quality controllability, Easy to operate, good method-specific effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
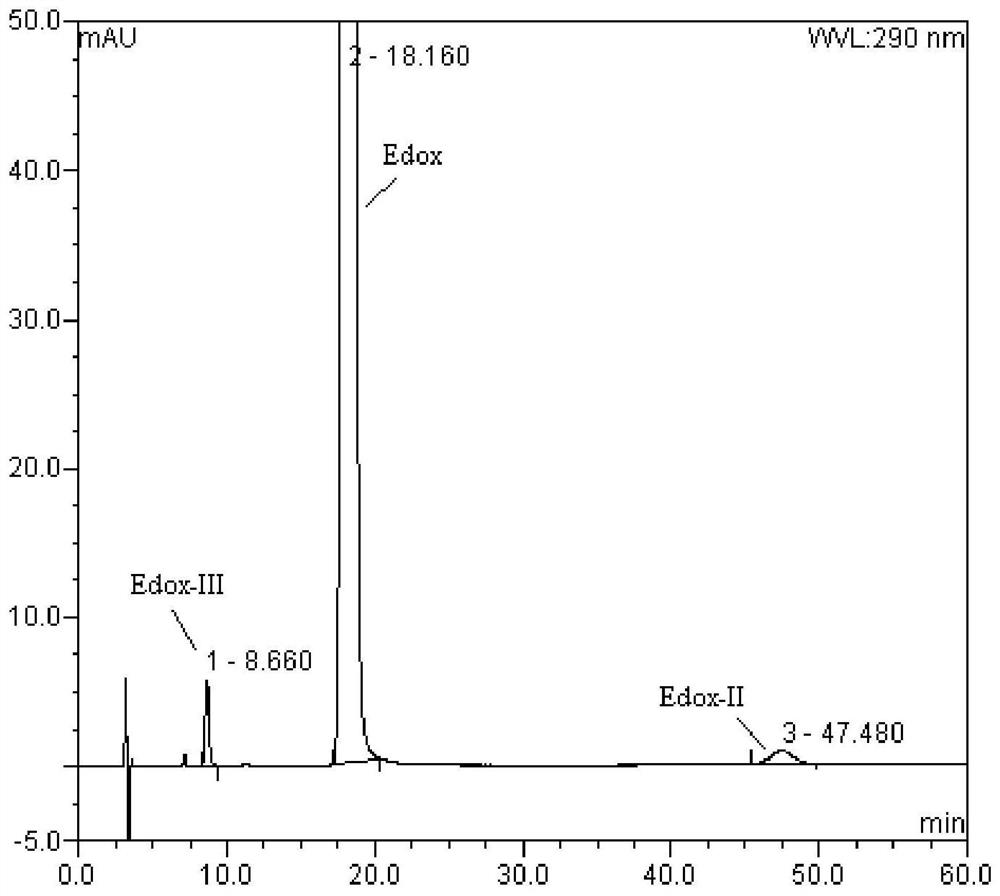
[0032] Example 1 Validation of the method for the determination of isomers Edox-II and Edox-III in edoxaban tosylate hydrate according to the present invention.
[0033] Chromatographic column: DAICEL CHIRALCEL OX-H chiral chromatographic column (size 4.6mm×250mm, 5μm);
[0034] Mobile phase: methanol-ethanol-diethylamine (40:60:0.3);
[0035] Detection wavelength: 290nm;
[0036] Flow rate: 1.0mL / min;
[0037] Column temperature: 35°C.
[0038] 1.1 System suitability test
[0039] Take an appropriate amount of Edox reference substance, Edox-II and Edox-III, add mobile phase to dissolve and make a mixed solution containing 1 mg of Edox, 5 μg of Edox-II and 5 μg of Edox-III per 1 ml, accurately measure 20 μl, and follow the above chromatographic conditions Injection analysis, the result shows: Edox-Ⅲ, Edox, Edox-Ⅱ peaks successively, and the resolution between the three is 15.5 and 13.3, reaching the baseline separation (see figure 1 ).
[0040] 1.2 Specificity test
[0...
Embodiment 2
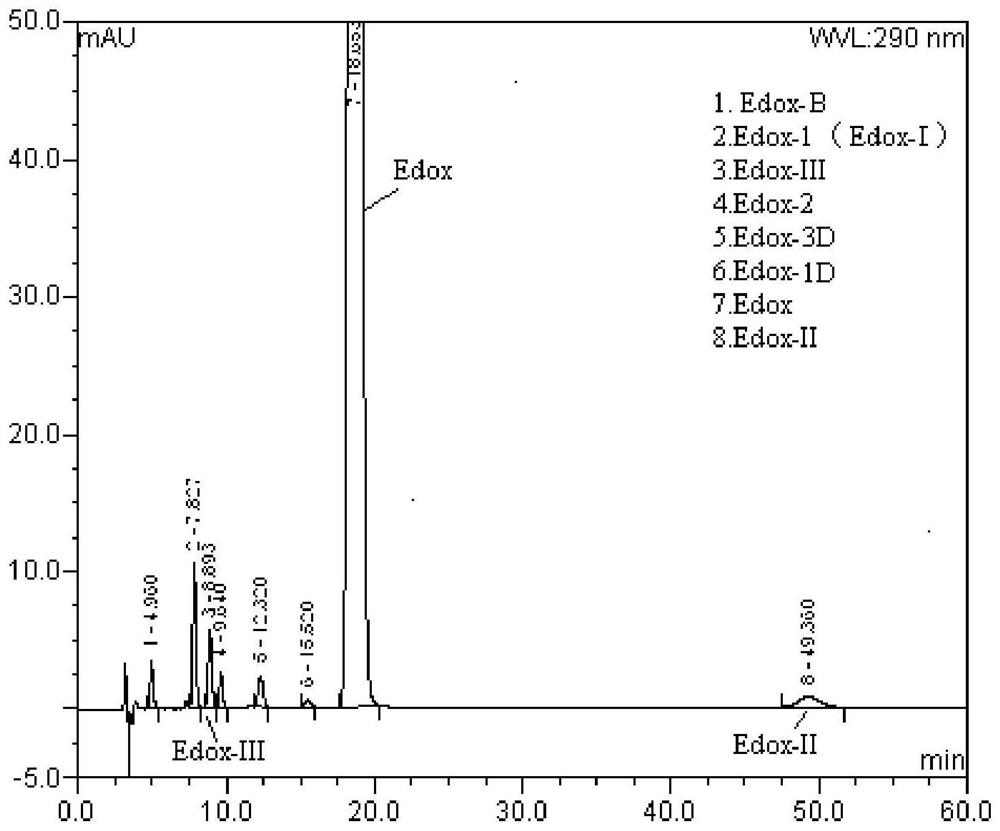
[0063] Example 2 Determination of isomers Edox-II and Edox-III in the Edoxaban Toluenesulfonate Hydrate Tablets of the present invention.
[0064] Take an appropriate amount of Edox reference substance, Edox-II and Edox-III, add mobile phase to dissolve and make a mixed solution containing 1 mg of Edox, 5 μg of Edox-II and Edox-III per 1 ml, as a system suitability solution. Take an appropriate amount of edoxaban tosylate hydrate tablet fine powder, accurately weighed, add mobile phase to dissolve and dilute to make a solution containing about 1.0 mg of edoxaban tosylate hydrate per 1 ml, shake well, filter Then, as the test solution, accurately measure 1.0ml, put it in a 100ml measuring bottle, dilute to the mark with mobile phase, shake well, and use it as a control solution. Take an appropriate amount of blank excipients for Edoxaban Toluenesulfonate Hydrate Tablets, add mobile phase to dissolve and dilute to make a solution of corresponding concentration, as the blank exci...
PUM
| Property | Measurement | Unit |
|---|---|---|
| correlation coefficient | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com