Preparation method of key intermediate of edoxaban
An equation and reaction system technology, applied in the direction of organic chemistry, etc., can solve the problems of low reaction yield, poor stability of hydrochloride, affecting reaction progress, etc., to improve the reaction yield, improve the reaction yield and purity, and reduce the dosage. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]
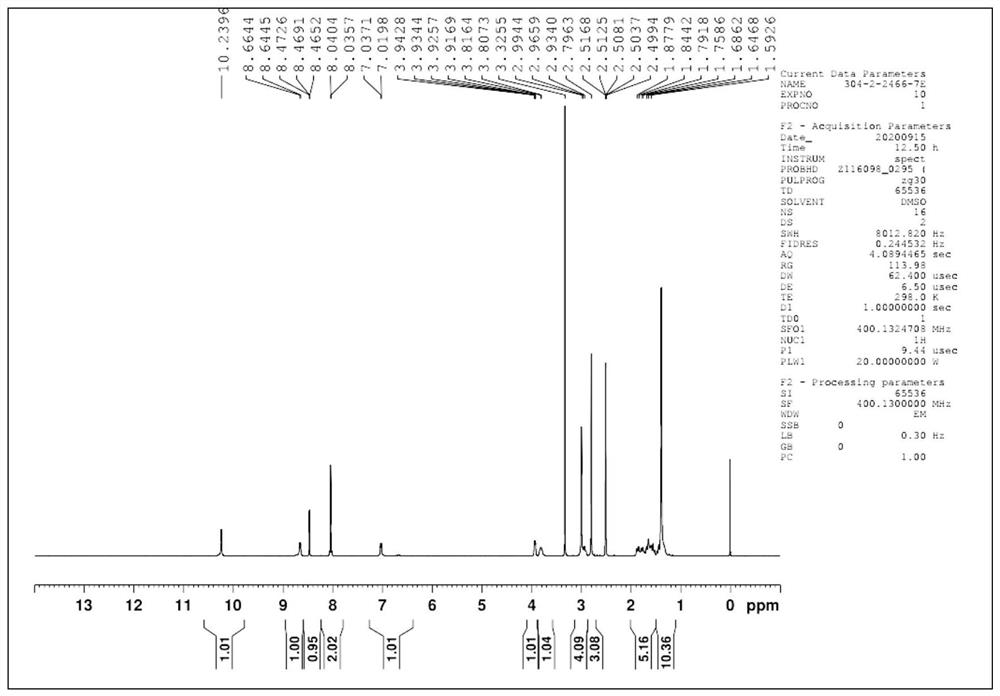
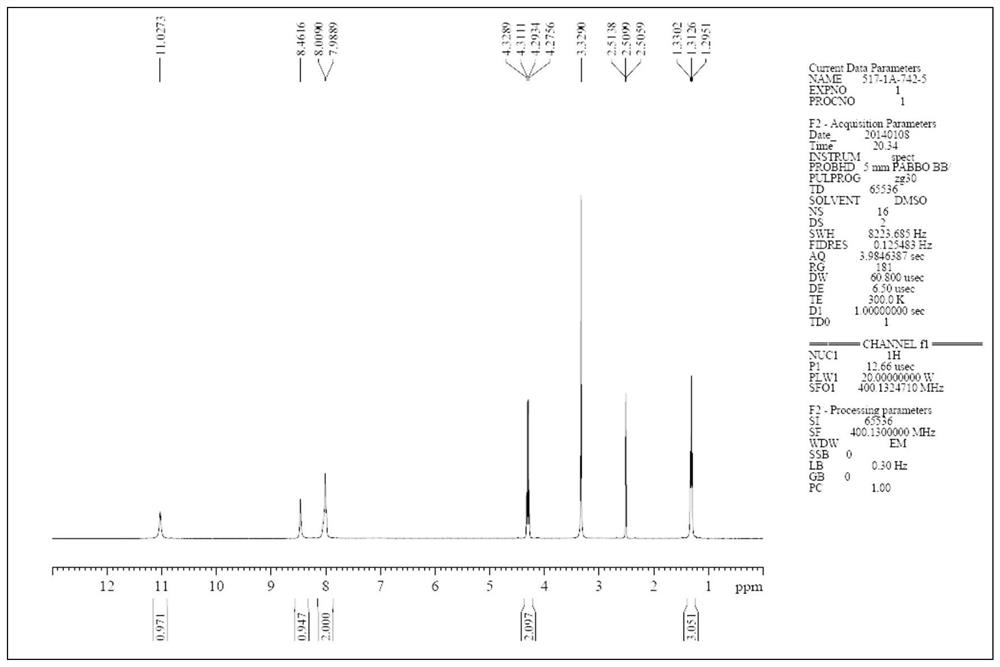
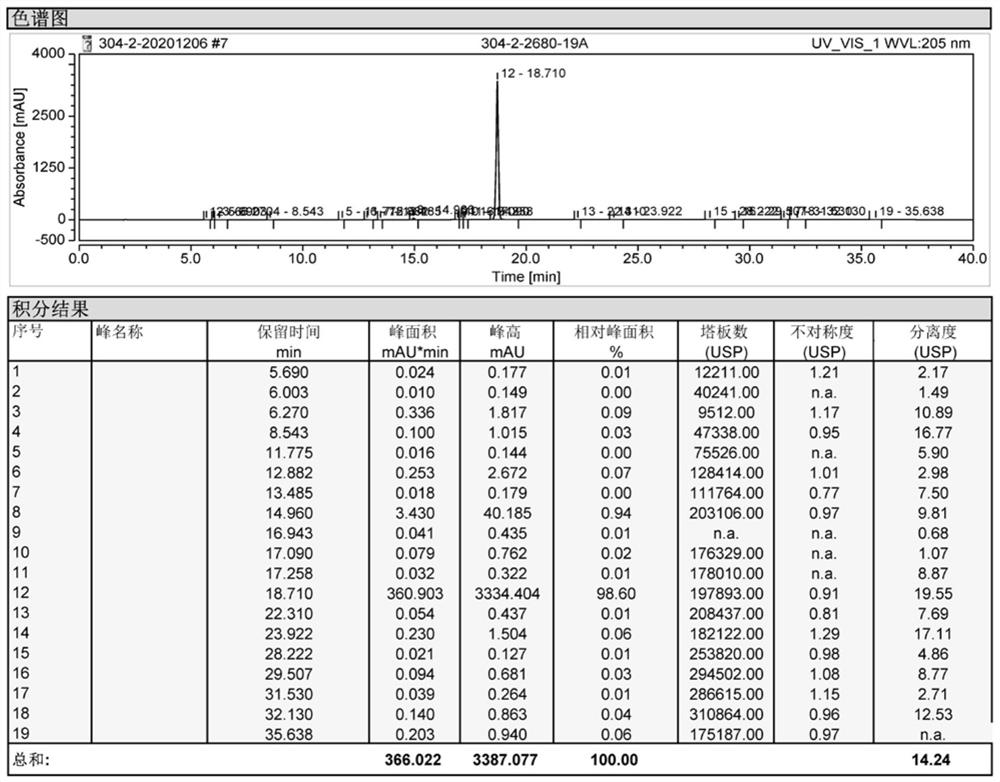
[0040] DMF (120g) was placed in a 500mL reaction flask, followed by adding compound A (22.5g, 0.079mol, 1.00eq.), compound B (18.9g, 0.083mol, 1.05eq.) and sodium acetate (6.5g, 0.079mol , 1.00eq.), heated to 95 ° C for 8h. Add 240 g of water, keep it at 85°C for 1 hour, solids precipitate out, filter with suction, rinse the filter cake with water, and dry to obtain 32.5 g of compound I as an off-white solid, with a yield of 88.1% and a purity of 98.2%.
Embodiment 2
[0042]
[0043] DMF (120g) was placed in a 500mL reaction flask, followed by adding compound A (22.5g, 0.079mol, 1.00eq.), compound B (18.9g, 0.083mol, 1.05eq.) and sodium acetate (9.7g, 0.118mol , 1.50eq.), heated to 90 ° C for 7h. Add 240g of water, keep it at 85°C for 1 hour, solids precipitate out, filter with suction, rinse the filter cake with water, and dry to obtain 34.3g of compound I as an off-white solid, with a yield of 93.0% and a purity of 98.9%.
Embodiment 3
[0045]
[0046] DMF (120g) was placed in a 500mL reaction flask, followed by adding compound A (22.5g, 0.079mol, 1.00eq.), compound B (18.9g, 0.083mol, 1.05eq.) and sodium acetate (19.4g, 0.236mol , 3.00eq.), heated to 85 ° C for 6h. Add 240g of water, continue to keep warm at 85°C for 1h, solids precipitate out, filter with suction, rinse the filter cake with water, and dry to obtain 34.1g of compound I as off-white solid, yield: 92.5%, purity: 98.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com