Axitinib fumarate, crystal form thereof and preparation methods for Axitinib fumarate and crystal form thereof
A technology of nifumarate and axitinib, applied in the field of medicinal chemistry, can solve the problems of high heavy metal palladium residue, high organic impurity content, poor appearance and the like in axitinib, and achieve good photostability and palladium residue. reduced, good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061]Embodiment 1: Preparation of axitinib fumarate
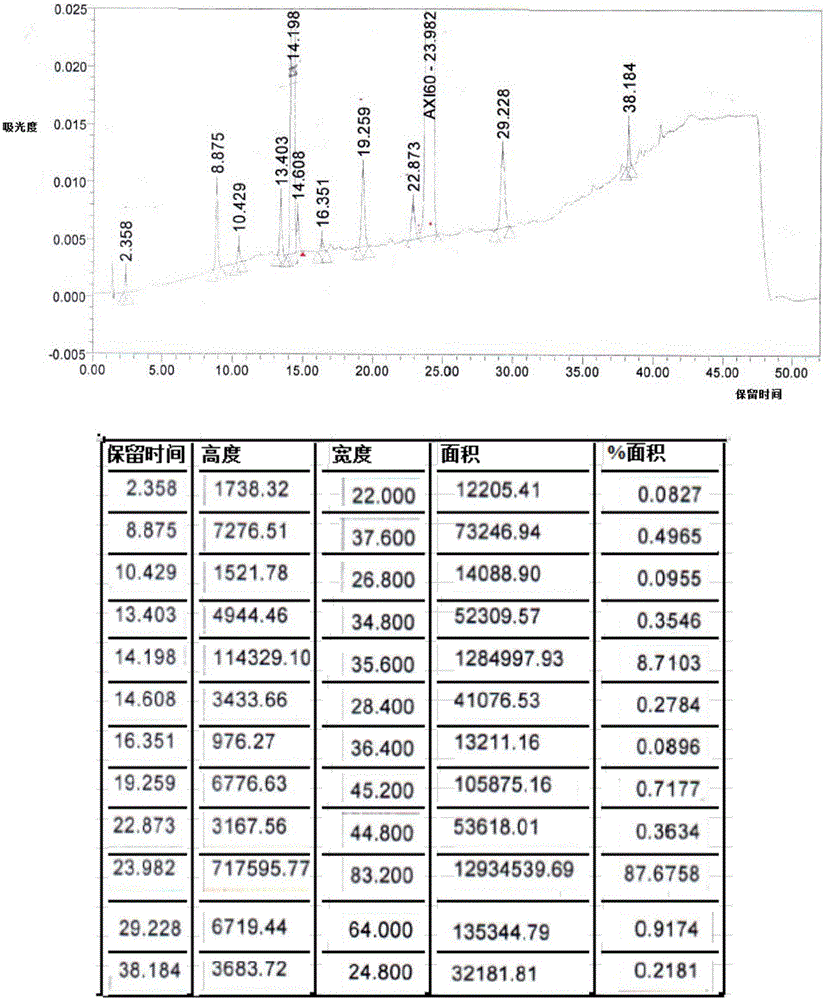
[0062] Add 150ml of ethanol and axitinib crude product at normal temperature (according to Organic Process Research & Development 2014, 18, 266-274, HPLC purity 87.7%, HPLC detection data are as follows Table 1 or see figure 1 , palladium residue 131ppm) (10g, 0.0259mol) into the reaction flask, under stirring, drop into fumaric acid (3.3g, 0.0284mol); warm up to 65~75°C and stir for 1 hour; then cool to room temperature, and carry out suction filtration After the suction filtration, the wet product was put into 50-60°C for vacuum drying to obtain 10.8g of axitinib fumarate, with a yield of 83%, HPLC 96.1%, and residual palladium of 11.5ppm. The HPLC detection data are shown in Table 2 or See figure 2 .
[0063] Table 1
[0064] keep time high width area %area 2.358 1738.32 22.000 12205.41 0.0827 8.875 7276.51 37.600 73246.94 0.4965 10.429 1521.78 26.800 14088.90 0.0955 13....
Embodiment 2
[0070] Embodiment 2: the preparation of axitinib fumarate
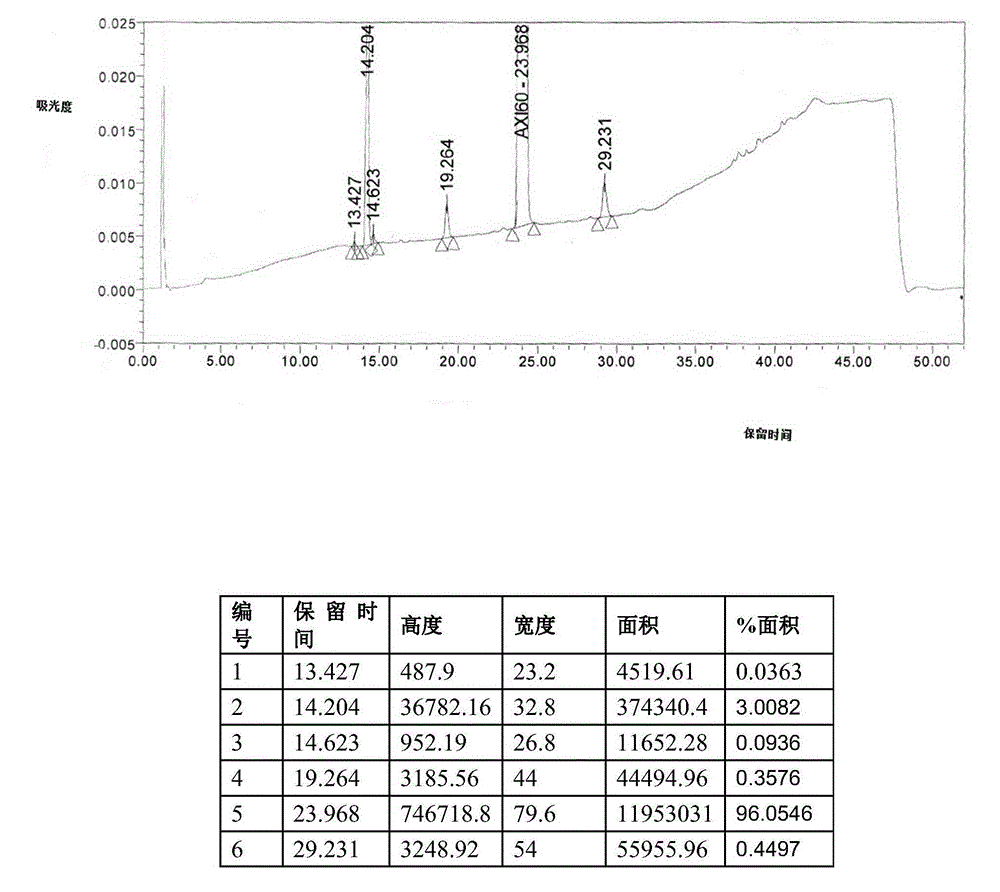
[0071] At normal temperature, 150ml of isopropanol and axitinib (according to Organic Process Research & Development 2014, 18, 266-274, HPLC purity 97.7%, HPLC detection data are as follows in Table 3 or see image 3 , palladium residue 65.2ppm) (10g, 0.0259mol) into the reaction flask, under stirring, drop into fumaric acid (3.3g, 0.0284mol); heat up to 65~75°C and stir for 1 hour; then cool to 0°C, and carry out After suction filtration, the wet product was placed at 50-60°C for vacuum drying to obtain 12.8 g of axitinib fumarate, with a yield of 98.4%, HPLC of 99.9%, and residual palladium of 5.7 ppm. HPLC detection data is as follows table 4 or see Figure 4 .
[0072] table 3
[0073] keep time high width area %area 2.340 1960.44 23.600 14980.44 0.1019 8.648 3934.0535.2 00 44757.95 0.3044 13.225 5017.56 40.800 57950.38 0.3941 13.929 2679.38 35.200 31555.30 0.21...
Embodiment 3
[0078] Embodiment 3: Preparation of axitinib fumarate in crystalline form (using ethanol and NNP as solvent)
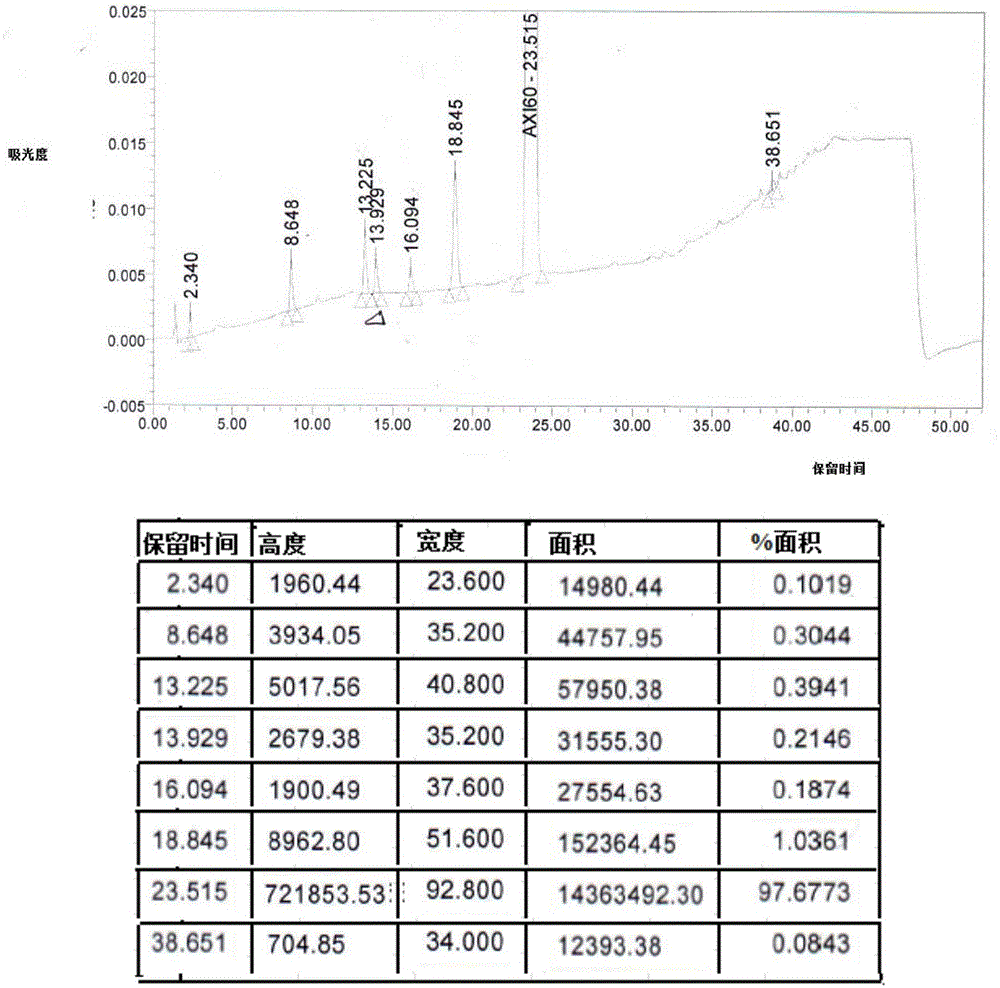
[0079] Add 150ml of ethanol, 10ml of N-methylpyrrolidone and crude axitinib at room temperature (according to Organic Process Research & Development 2014, 18, 266-274, HPLC detection data are as follows in Table 5 or see Figure 5 , HPLC purity 97.7%, palladium residual 69.6ppm) (10g, 0.0259mol) into the reaction flask, under stirring, drop into fumaric acid (3.3g, 0.0284mol); be heated to 65~75 ℃ and stir for 1 hour; then cool to room temperature ℃, carry out suction filtration on it, after the suction filtration, put the wet product into vacuum drying at 50℃~60℃ and 80℃~100℃ respectively, and see Figure 8 It is the ethanolate of axitinib fumarate in vacuum (drying temperature is 50 ℃ ~ 60 ℃), see Figure 9 It is Form A of axitinib fumarate (vacuum drying temperature is 80°C to 100°C). A total of 13.0 g of axitinib fumarate was obtained, with a yield of 99.9%, HPL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com