Enteric omeprazole micropill and its preparing method
A technology of omeprazole intestines and omeprazole, which is applied in the directions of pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., can solve the problem of the amount and release rate affecting the release of active ingredients. Uniformity of drug release, adverse health effects of patients taking the drug, electrostatic adsorption of particles, etc., to achieve the effects of low cost, dense coating, rapid and stable release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The present embodiment omeprazole enteric-coated pellets constitute the following table:
[0029] ingredients
Content (wt%)
Inner active ball core
Omeprazole
8.0
58.0
3.2
1.6
HPMC (viscosity 4)
0.08
Intermediate isolation layer
HPMC (viscosity 4)
PEG-6000
Titanium dioxide
8.8
1.8
1.7
outer enteric coating layer
Eudragit L100-55
PEG-6000
12.4
1.0
3.42
[0030] Preparation:
[0031](1) prepare 1% HPMC solution: soak HPMC with 70 ℃ hot water 45min, then stir well, make 1% solution; Mix the magnesium oxide evenly, divide it into 2 parts, spray one part into 1% HPMC solution to prepare pellets with a diameter of 0.2-0.3mm, and the other part for later use; In the machine, continue to spray 1% HPMC solution, and at the same time, add the r...
Embodiment 2
[0037] The present embodiment omeprazole enteric-coated pellets constitute the following table:
[0038] ingredients
Content (wt%)
Inner active ball core
Omeprazole
8.0
49.0
9.6
3.2
HPMC (viscosity 4.5)
0.05
Intermediate isolation layer
HPMC (viscosity 4.5)
PEG-6000
Titanium dioxide
8.5
0.8
1.8
outer enteric coating layer
Eudragit L100-55
PEG-6000
14.9
1.3
2.85
[0039] Preparation:
[0040] With embodiment 1.
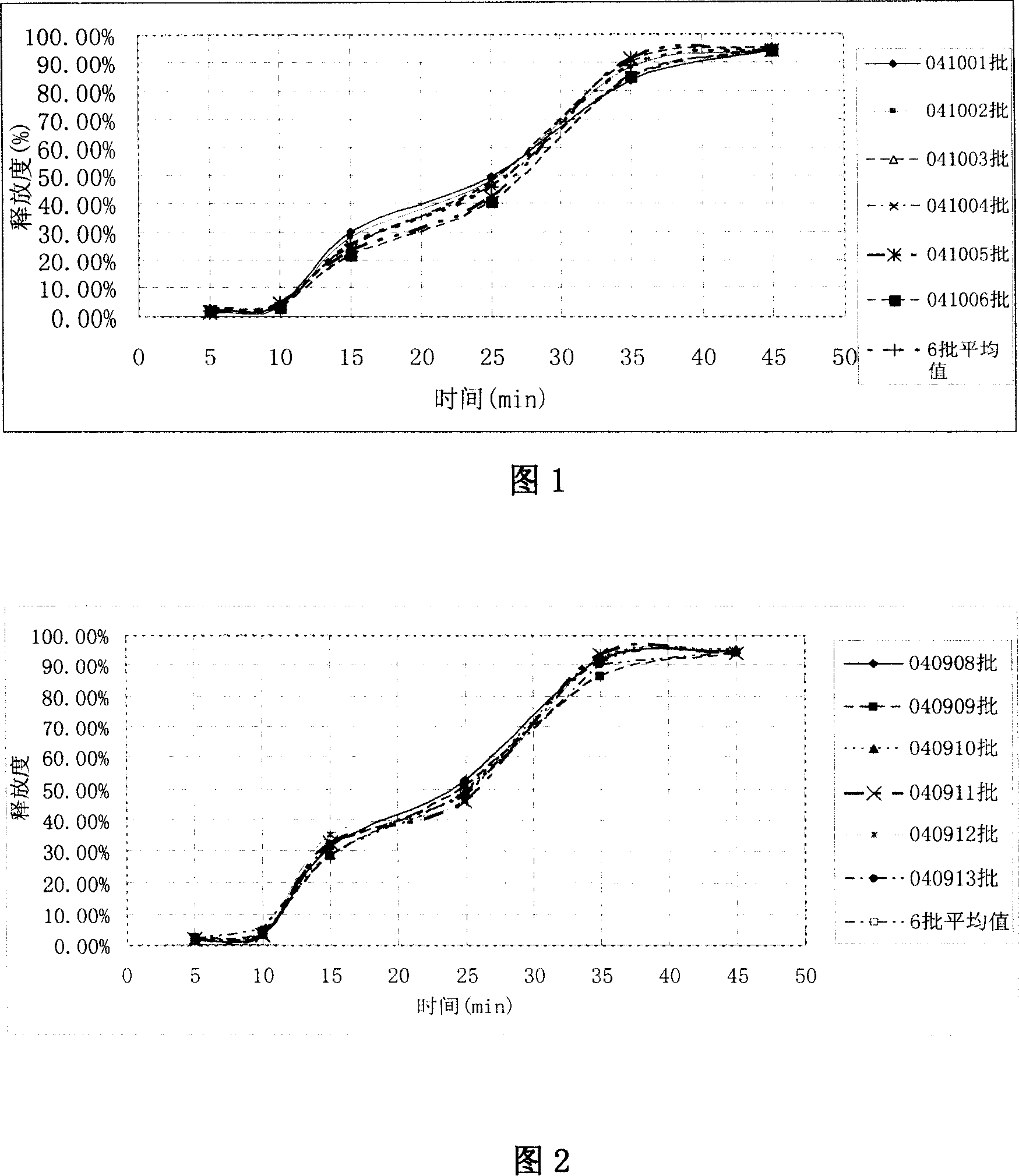
[0041] The seven graphs shown in Figure 2 represent the release of different batches of omeprazole enteric-coated pellets and capsules of Example 2. The graph shows the release at different times in an alkaline environment, and the release curve with the average of 6 batches of release. This figure further proves that the method of the presen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com