Novel antitumoral use of cabazitaxel
A technology of cabazitaxel and compounds, applied in the field of novel anti-tumor applications, which can solve problems such as limiting treatment options
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
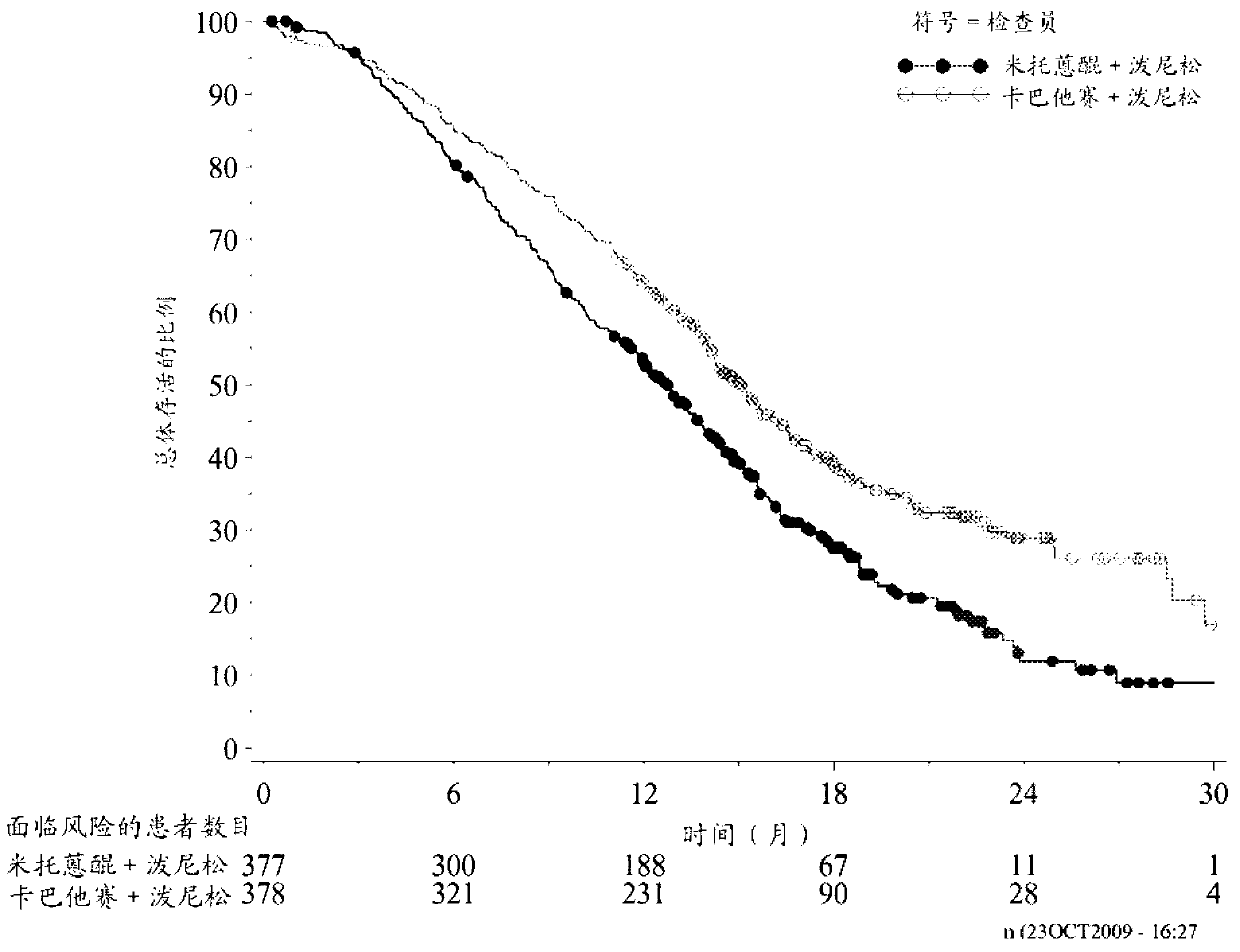
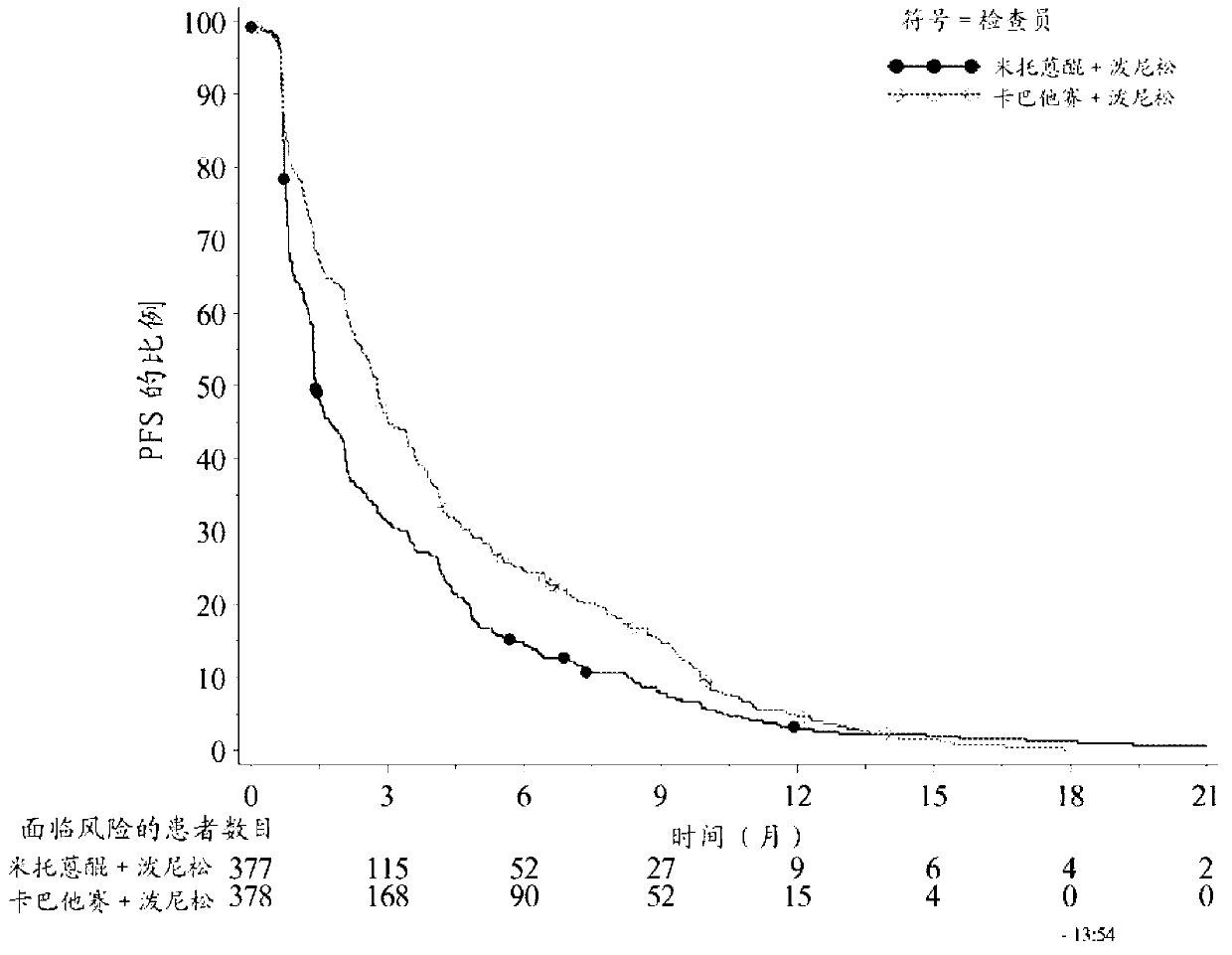
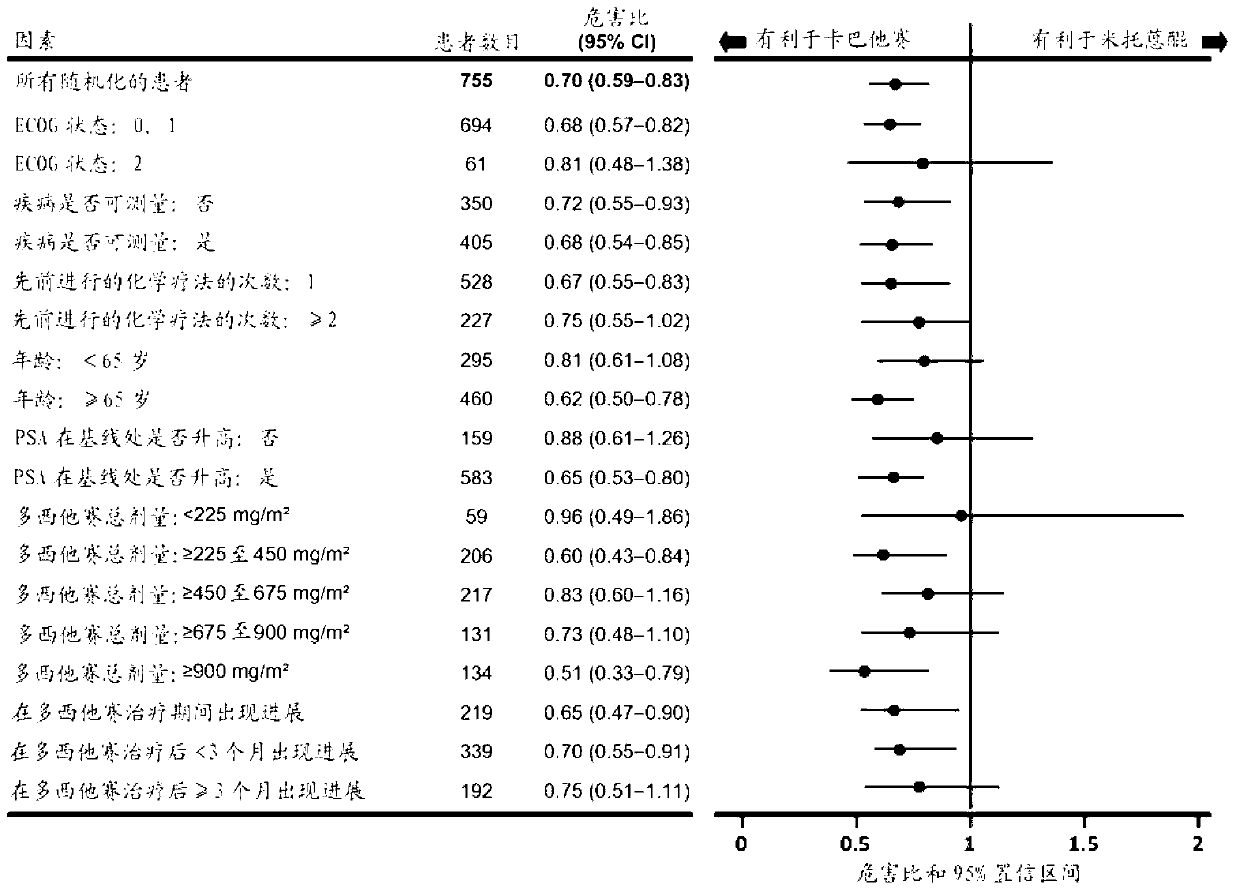
[0111] A clinical study was performed in which patients received treatment with cabazitaxel or a reference treatment based on mitoxantrone, each in combination with prednisone or prednisolone.
[0112] More specifically, the following patients were randomized to receive 10 mg / day prednisone + 12 mg / m 2 Mitoxantrone or 10mg / day prednisone+25mg / m 2 Of cabazitaxel, in which mitoxantrone and cabazitaxel are both administered once every 3 weeks, the patient is over 18 years old, suffering from metastatic castration-resistant metastatic prostate cancer, the prostate cancer can be passed RECIST standard to measure or not measurable, in which PSA levels increase or new lesions appear, the Eastern Cooperative Oncology Group (ECOG, Eastern Cooperative Oncology Group) physical status is 0-2 and the organ function is appropriate (patients must have neutrophils> 1,500 cells / mm 3 , Platelet> 100,000 cells / mm 3 , Hemoglobin> 10g / dL, creatinine 2 ) During or during docetaxel treatment (cumulative ...
Embodiment 2
[0161] Table 6 shows examples of dose adjustments for adverse reactions in patients treated with cabazitaxel.
[0162] Table 6
[0163]
[0164] If the patient is at a dose of 20mg / m 2 If you continue to experience any of these reactions, stop cabazitaxel treatment.
Embodiment 3
[0166] Physical state and pain score during treatment
[0167] method
[0168] -ECOG PS, pain measurement and analgesic consumption are evaluated before each treatment cycle and at the end of study treatment.
[0169] -Pain evaluation: current pain intensity (PPI) scale from the McGill-Melzack questionnaire (Melzack R. Pain 1975; 1:277-99). The average analgesic score (AS) for the week before each evaluation was calculated, which was derived from the consumption of analgesics (based on the equivalent of morphine). The area under the curve (AUC) of PPI and AS is calculated by the trapezoidal formula. For each patient, the cumulative AUC of PPI and AS is calculated to the last cycle for which data is available. From the first cycle to the tenth cycle, the average AUC of each treatment group was compared.
[0170] result
[0171] -The physical status of most patients remained stable during treatment and was similar between groups. See Figure 4 .
[0172] -Overall, the PPI scores are...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com