Glucocorticoid synergist
A technology of glucocorticoid and synergist, which is applied in the field of medicine to achieve the effects of enhancing anti-inflammation, enhancing biological effects, and reducing incidence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1. In vitro experiment:
[0034] 1. With different concentrations of GS (6.25-100 mg / L) and 10 -8 HL-7702 cells were co-treated with M / L Dex, and the effect of GS on GC-induced GR transcriptional activation was detected after 24 hours.
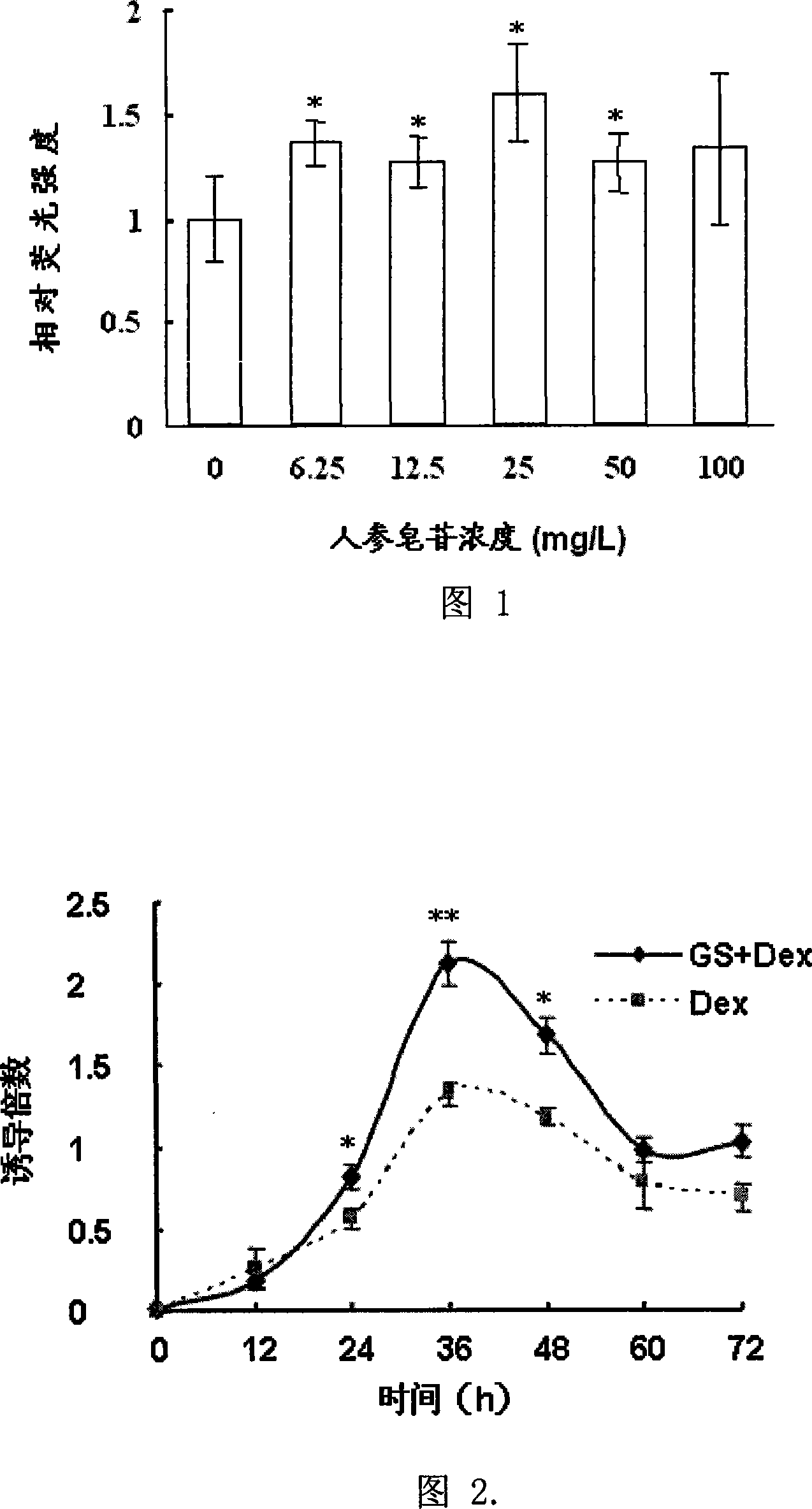
[0035] Figure 1 shows the dose-effect relationship of GS affecting GC-induced reporter gene expression. It can be seen that GS has a significant enhancement effect on the expression of GR reporter gene induced by GC, but it is not dose-dependent. When the concentration of GS is 25mg / L, the enhancement effect of GS is the strongest.
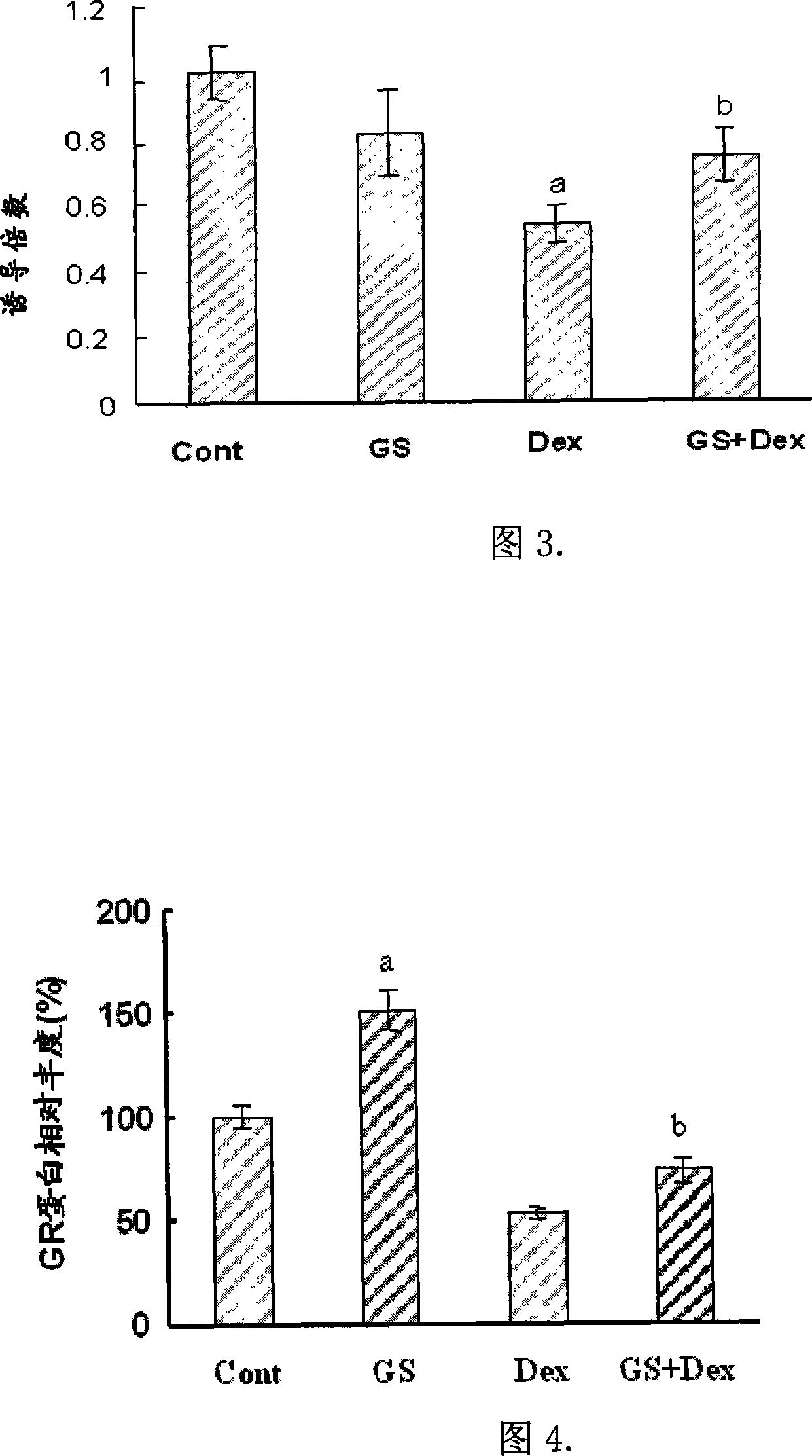
[0036] Figure 2 shows the time-dependent relationship between GS and GC-induced reporter gene expression. It can be seen that GS can significantly enhance the GC-induced GR transcriptional activation effect. When the dose of GS is 25mg / L and the action time is 36h, its effect is the best.
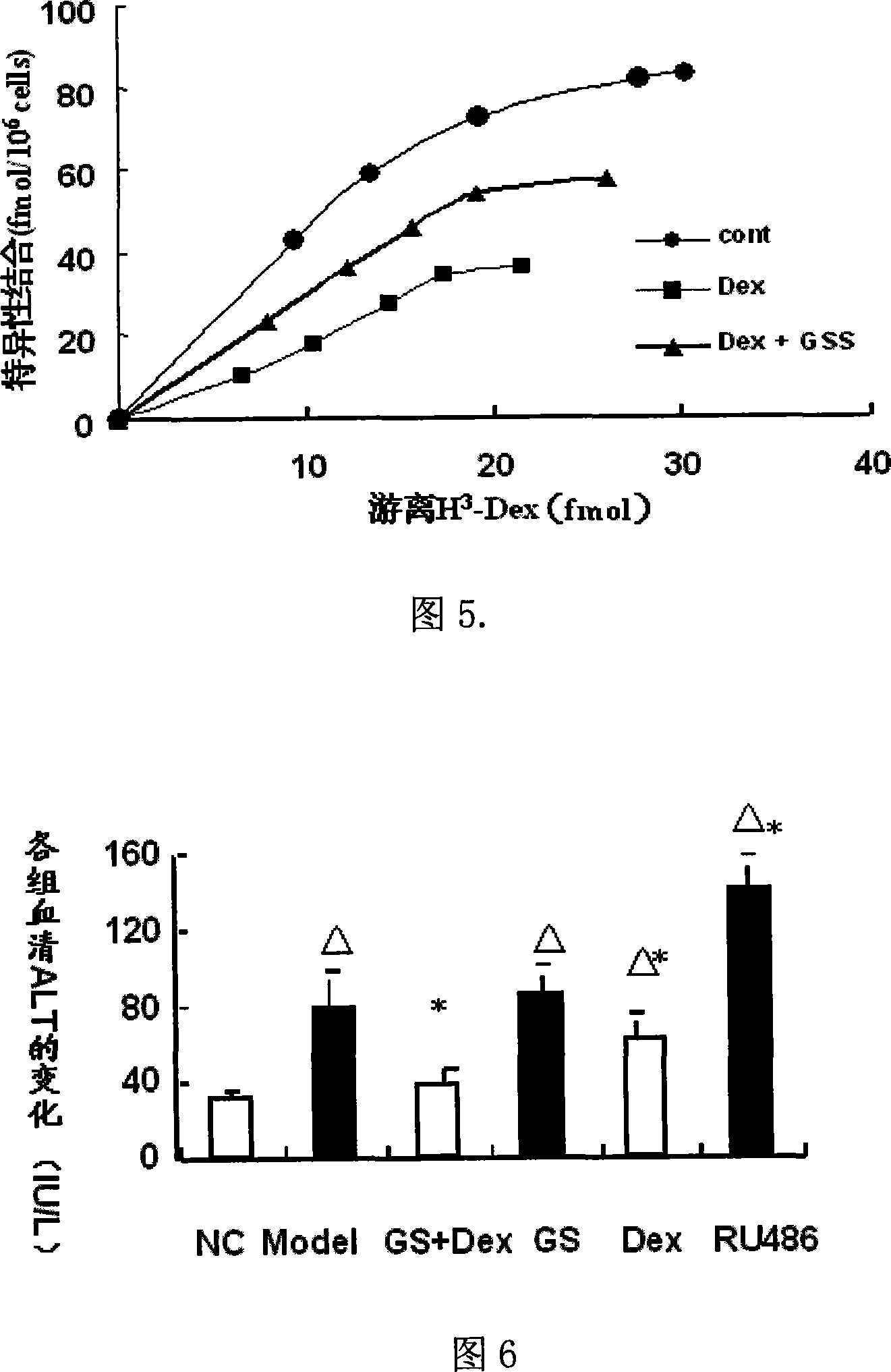
[0037] 2. Contain 25 mg / L GS and Dex (10 -8 mol / L) culture medium to induce the cells for 36 hours, the...
Embodiment 2
[0048] Embodiment 2. Animal experiments:
[0049] 1. To observe the protective effect of different concentrations of GS combined with Dex on liver function in common hepatic artery ligation model rats. We chose postoperative 12h, the time point with the most severe liver function damage, as the observation point, and found that GS had synergistic effects on Dex (5 mg / kg) in the range of 10-100 mg / kg, and the increase of 50 mg / kg GS The effect was the best, so this concentration and dosage was selected for further research.
[0050] Figures 6-8 show the enzymatic changes of liver function in rats in each group 12 hours after the ligation of the common hepatic artery. The results showed that ALT and ALP in the GS combined with Dex group could return to the level of the normal control group (P>0.05), and AST was still higher than the normal control group (P<0.01), but significantly lower than the model group (P<0.01); The levels of ALT and AST in the model group were lower than...
Embodiment 3
[0055] Embodiment 3. clinical trial:
[0056] Clinical experiments have confirmed that GS can enhance the anti-inflammatory, anti-stress and immunosuppressive effects of GC. TranscatheterArterial Chemoembolization (TACE) is an invasive operation, during which patients will experience both psychological and physical stress, and at the same time, the chemotherapeutic drugs and embolic agents infused through the artery will cause liver tissue ischemia necrosis, resulting in an inflammatory response. Therefore, TACE is a good model for studying anti-inflammation and anti-stress. In terms of verifying the enhanced GC immunosuppressive effect, patients with various autoimmune diseases such as systemic lupus erythematosus, nephrotic syndrome, and polymyositis will be selected as research objects.
[0057] 1. Using a randomized double-blind control design, according to the inclusion and exclusion criteria, 120 patients with primary liver cancer who were hospitalized in the Departmen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com