Compound preparation for treating nephrotic syndromes
A technology for nephrotic syndrome and compound preparations, applied in the field of medicine, can solve problems such as inconspicuous infection symptoms, spread of infection, and aggravation of nephrotic syndrome bone disease, and achieve the effects of enhancing biological effects, enhancing biological effects, and controlling disease progression.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Scheme 1 of the present invention (group A of compound preparation): 400 mg of ginsenoside and 10 mg of prednisone.
[0013] Scheme 2 of the present invention (compound preparation B group): ginsenoside 400 mg, methylprednisone 8 mg.
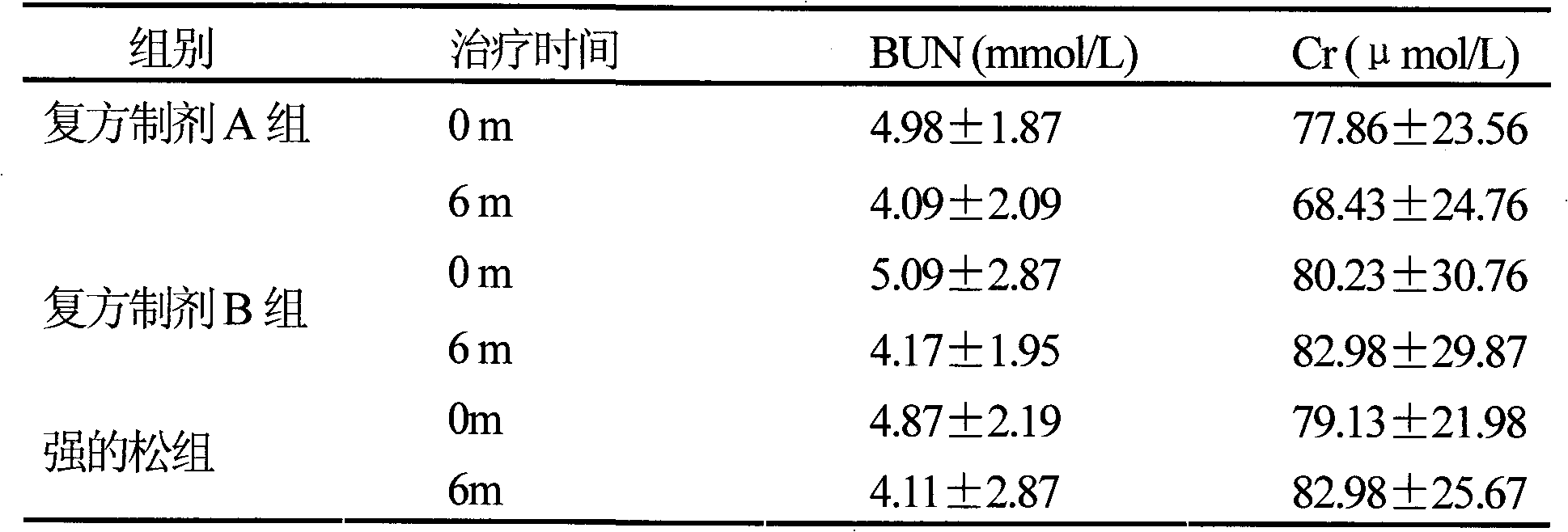
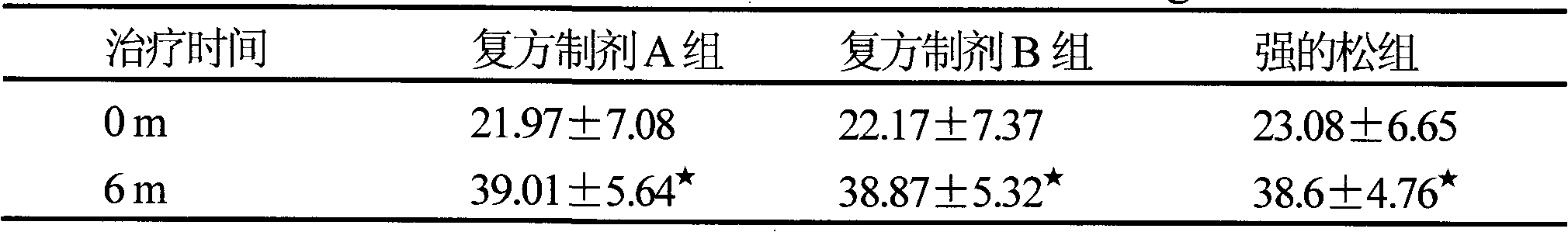
[0014] It has been confirmed by clinical trials that the compound preparation can reduce the dosage of hormones to achieve the same therapeutic effect.
[0015] A randomized double-blind control design was used to collect 180 patients with nephrotic syndrome who were outpatients and inpatients in the Department of Nephrology according to the inclusion and exclusion criteria. Randomly divided into three groups:
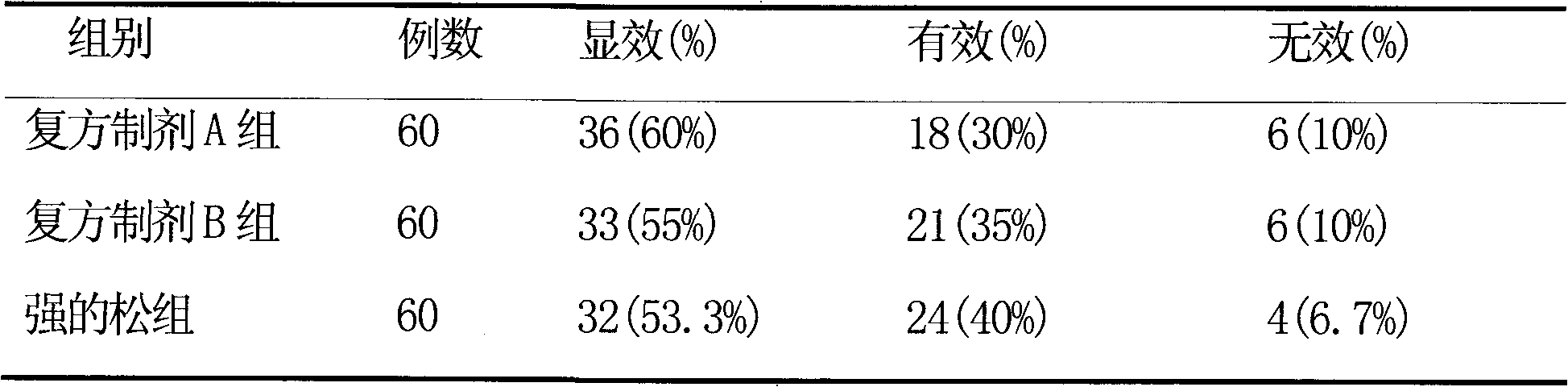
[0016] Prednisone routine treatment group, given prednisone 15mg orally, once a day;
[0017] Compound preparation group A was given a compound preparation containing 400 mg of ginsenoside and 10 mg of prednisone orally, once a day;
[0018] The compound preparation group B was given a compound preparation containing 400 mg of ...
Embodiment 2
[0043] Scheme 3 of the present invention (compound preparation C group): ginsenoside 400mg and prednisone 20mg,
[0044] Scheme 4 of the present invention (compound preparation D group): ginsenoside 400mg and methylprednisone 16mg;
[0045] It is confirmed by clinical trials that the compound preparation can improve the curative effect of nephrotic syndrome.
[0046] A randomized double-blind control design was adopted, and 90 patients with nephrotic syndrome who visited the nephrology outpatient clinic or were hospitalized in the ward were collected according to the inclusion and exclusion criteria. They were randomly divided into three groups: prednisone routine treatment group, oral prednisone 20mg once a day; compound preparation C group, compound preparation containing ginsenoside 400mg and prednisone 20mg orally once a day; compound preparation D Group, given the compound preparation containing ginsenoside 400mg and methylprednisolone 16mg orally once a day. The curati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com