Application of chlorogenic acid in preparation of medicines for treating osteopetrosis
A technology of osteosclerosis and chlorogenic acid, applied in bone diseases, drug combinations, pharmaceutical formulations, etc., can solve the problems of poor treatment effect, large toxic and side effects, no reports of chlorogenic acid osteosclerosis, etc., and achieve clinical results. Good application prospect and less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1: In vivo pharmacodynamics study of chlorogenic acid in the treatment of osteosclerosis
[0017] 1. Experimental materials
[0018] 1.1 Animals
[0019] Fifty BALB / C mice with osteopetrosis.
[0020] The animal model used in this example is directly purchased BALB / C mice with osteopetrosis (this mouse is also a commonly used model for basic research on osteopetrosis at home and abroad). In this mouse model, due to the abnormal growth of the bone in the sick mouse, the bone is hard, the bone marrow cavity disappears, all the bones are hardened, and there is edentulousness. The lesions of this mouse are similar to human osteosarcoma.
[0021] 1.2 Experimental Drugs and Instruments
[0022] Chlorogenic acid, prednisone (Hebei Xinyinhe Chemical Co., Ltd.), Shujin Huoxue Tablets (Henan Zhongjie Pharmaceutical Co., Ltd.), double distilled water, 12-well sterile plate, 96-well sterile plate, high power microscope, Electronic balance (one hundred thousandth).
[...
Embodiment 2
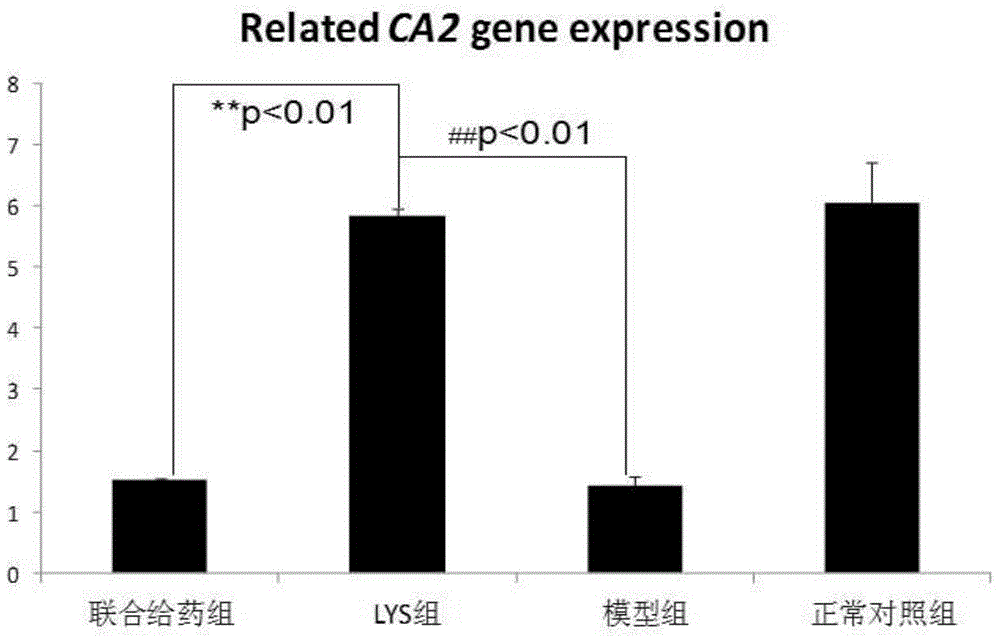
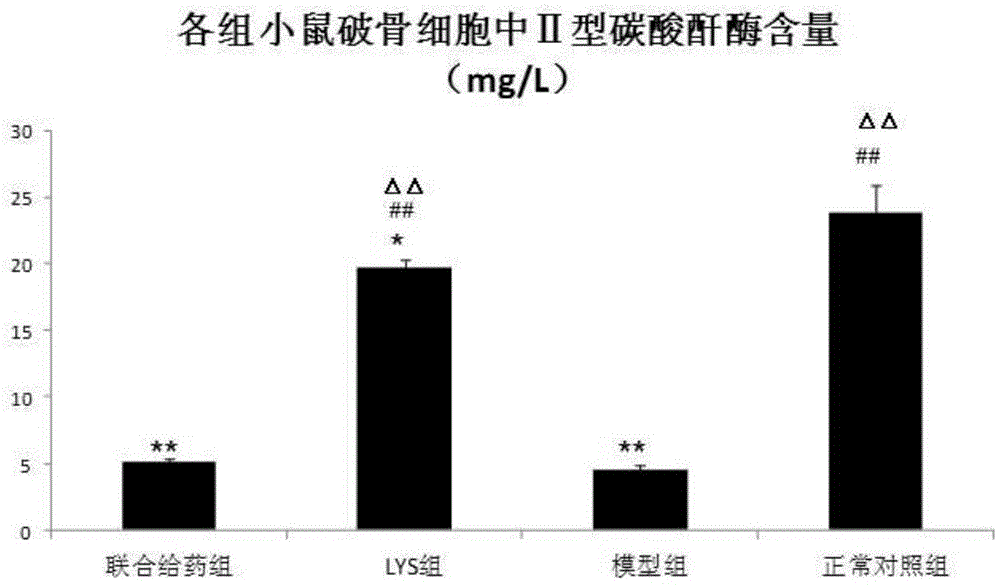
[0058] Example 2: Effect of chlorogenic acid on the expression of CA2 gene and its corresponding type II carbonic anhydrase in osteoclasts of a mouse model of osteosclerosis.
[0059] 1. Experimental materials
[0060] 1.1 Animals
[0061] Thirty BALB / C mice with osteopetrosis.
[0062] The animal model used in this example is directly purchased BALB / C mice with osteopetrosis. In this mouse model, due to the abnormal growth of the bone in the sick mouse, the bone is hard, the bone marrow cavity disappears, all the bones are hardened, and there is edentulousness. The lesions of this mouse are similar to human osteosarcoma.
[0063] 1.2 Experimental Drugs and Instruments
[0064] Chlorogenic acid, prednisone (Hebei Xinyinhe Chemical Co., Ltd.), Shujin Huoxue Tablets (Henan Zhongjie Pharmaceutical Co., Ltd.), type Ⅱ carbonic anhydrase ELISA detection kit, PCR instrument, RNA extraction kit, cDNA Chain synthesis kit, double distilled water, 12-well and 96-well plate, micropla...
Embodiment 3
[0102] Embodiment 3: Prepare freeze-dried powder injection with chlorogenic acid
[0103] 1. Extraction of chlorogenic acid:
[0104] The chlorogenic acid crude drug used in this example is obtained by extracting and purifying Eucommia leaves, with a purity of 99.38%.
[0105] 2. Preparation of chlorogenic acid freeze-dried powder injection
[0106] 2.1 Prescription:
[0107] Chlorogenic acid (main drug) with a purity of 99.38%
[0108] Completely dissolve the above prescription in water for injection, filter, and fine filter with a 0.22 μm sterilizing microporous membrane, adjust the pH, and make a total of 1000 2ml powder injections according to the routine operation of sterile powder injections, each Contains chlorogenic acid 40mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com