Biodegradable rapamycin-prednisone composite medicinal coat metal stent
A metal stent and drug coating technology, used in stents, coatings, medical science and other directions, can solve the problem of stents prone to local inflammation, and achieve the effects of inhibiting smooth muscle cell proliferation, reducing surgical complications, and preventing restenosis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The base part of the metal stent described in this embodiment is laser-engraved with L605 cobalt-chromium alloy as an open-loop design shape.
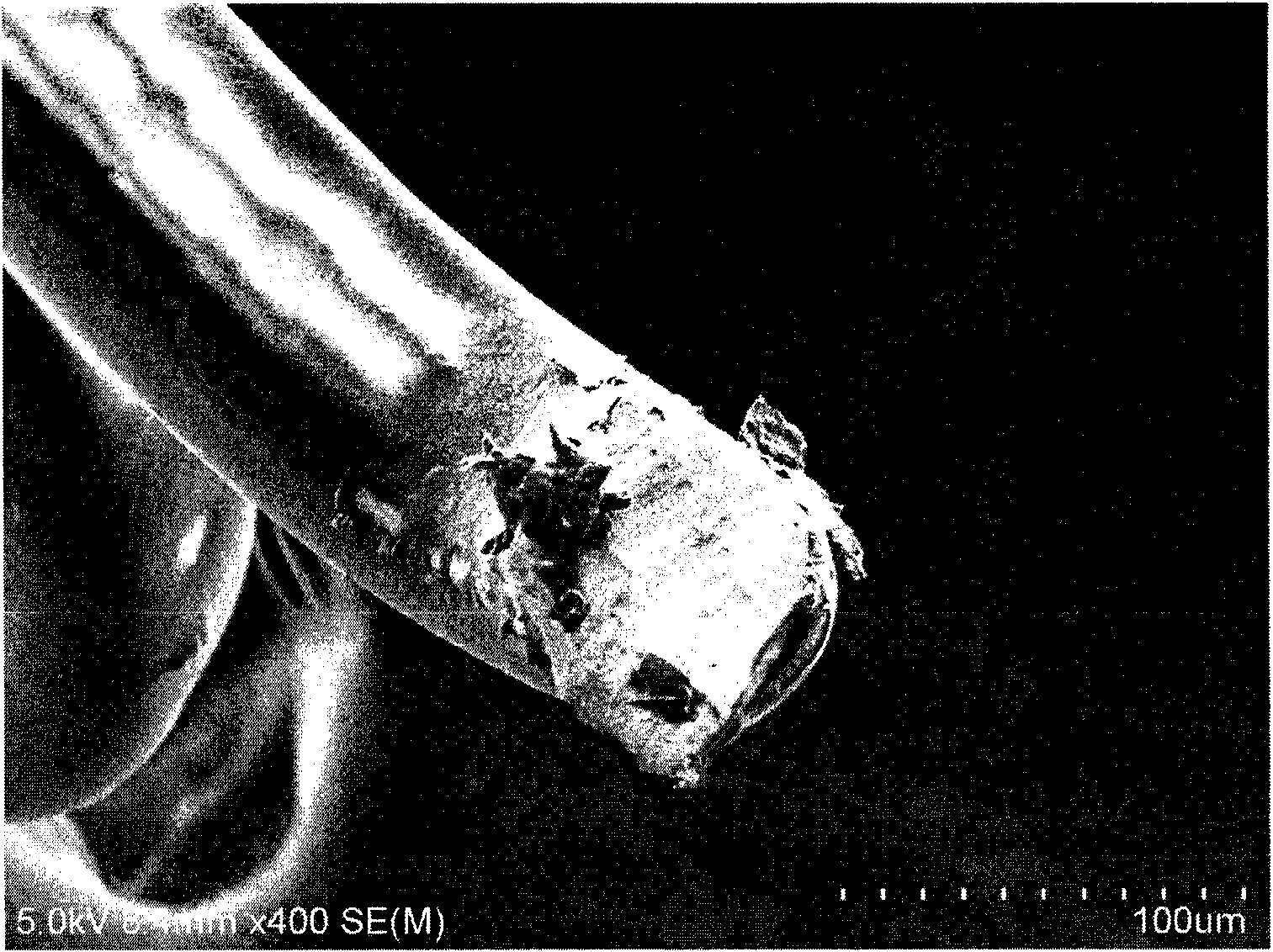
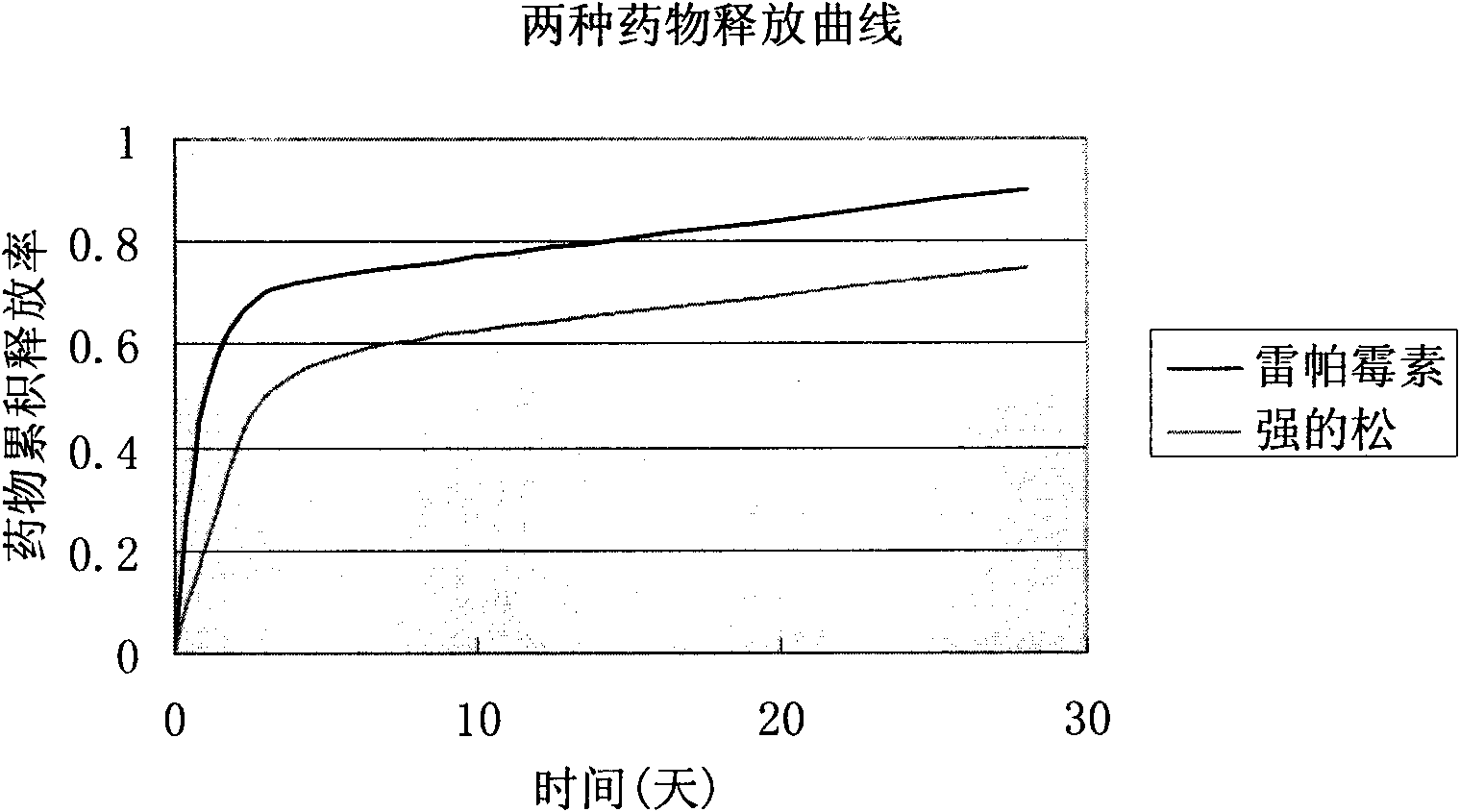
[0024] First get the mixture of rapamycin, prednisone and polyglycolide (PGA) respectively in the weight ratio of 1:1:3 and dissolve in trichloroethane, the total weight concentration is 1.5%, mix under room temperature Disperse evenly, and then ultrasonically spray on the surface of the metal stent. Cured in air for 30min. Repeat the above operation process until the drug loading of the stent reaches the requirement (rapamycin 1.6ug / cm 2 , Prednisone 1.0ug / cm 2 , coating thickness 5mm). The stent was then dried in a vacuum oven for 0.5 hours. figure 1 It is the scanning electron microscope image of the completed drug-coated stent, figure 2 A cross-sectional view of the coated stent.
[0025] Among them, polyglycolide (PGA) can be replaced by polylactide (PLA) or polycaprolactone (PCL), or a copolymer of two or three of t...
Embodiment 2
[0028] The base part of the metal bracket described in this embodiment is laser-engraved with 316L stainless steel into a hollow diamond-shaped structure.
[0029] First get the mixture of rapamycin, prednisone and polylactide (PLA) respectively by the weight ratio of 20:5:60 and dissolve in methanol, the total weight concentration is 2.0%, mix and disperse evenly at 40 ℃, then Ultrasonic spraying on the surface of the metal stent. Cured in air for 50min. Repeat the above operation process until the drug loading of the stent reaches the requirement (rapamycin 1.2ug / cm 2 , Prednisone 0.5ug / cm 2 , coating thickness 3mm). The scaffolds were then dried in a vacuum oven for 16 hours.
Embodiment 3
[0031] The base part of the metal stent described in this embodiment is laser-engraved with a nickel-titanium alloy into a "Z" corrugated shape.
[0032] First take the mixture of rapamycin, prednisone and polycaprolactone (PCL) respectively according to the weight ratio of 40:30:50 and dissolve them in THF, the total weight concentration is 3.0%, mix and disperse evenly at 60°C, Then it is ultrasonically sprayed on the surface of the metal stent. Cured in air for 30min. Repeat the above operation process until the drug loading of the stent reaches the requirement (rapamycin 2.0ug / cm 2 , Prednisone 1.0ug / cm 2 , coating thickness 1mm). The scaffolds were then dried in a vacuum oven for 32 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com