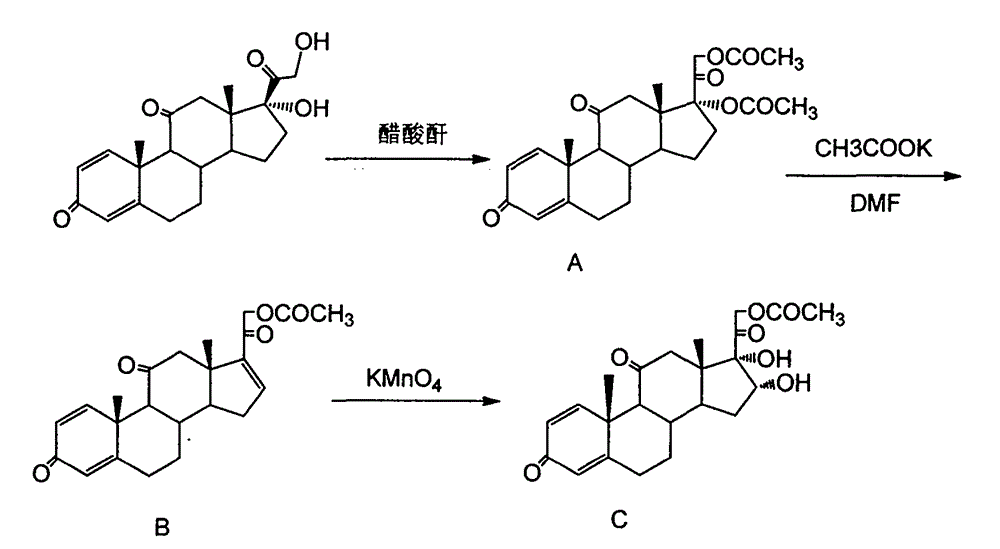
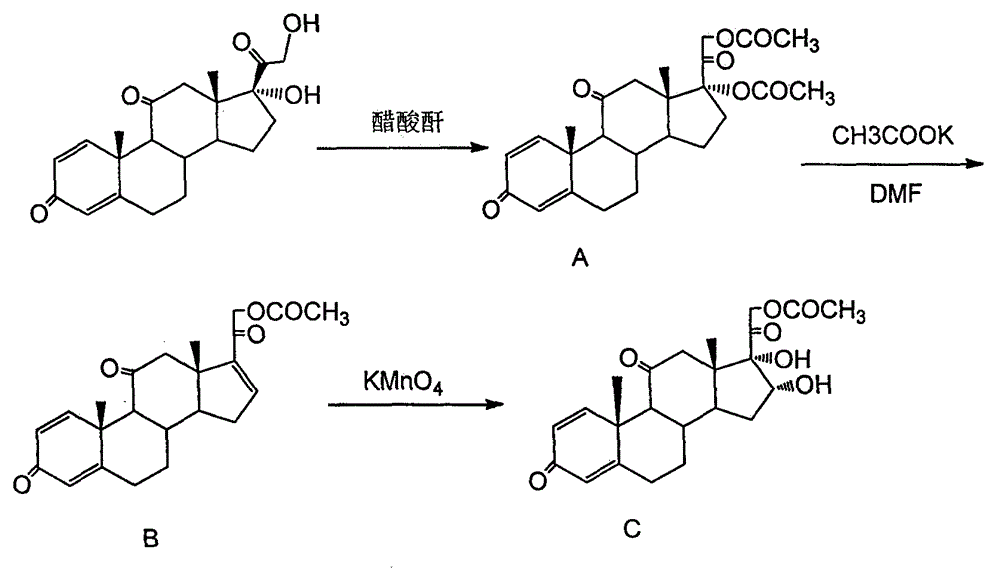
Preparation method of 16a,17a-dyhydroxyl-21-acetoxyl-1,4-pregnene diene-3,11,20-triketone
A kind of technology of gestodene and acetoxy, which is applied in the field of preparation of 16a,17a-dihydroxy-21-acetoxy-1,4-gestodene-3,11,20-trione, can Solve problems such as many side reactions, unpublished products complying with the European Pharmacopoeia, and complex products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] 1) In a 500ml dry reaction flask, add 35.8g raw material prednisone (0.1mol), 70ml ethyl acetate, 71.6ml acetic anhydride, 0.35g p-toluenesulfonic acid, install a reflux condenser, under nitrogen protection Stir and heat up and control the temperature at 70°C for 2h. After the reaction was detected by TLC, it was cooled to room temperature, ethyl acetate was evaporated, then washed with 100ml of water, filtered, and the filter cake was dried at low temperature to obtain a crude product, which was separated and purified to obtain a white solid powder A (17a, 21-diacetoxy- 1,4-pregnadiene-3,11,20-trione) 42g, yield 95%, mp: 225-226°C.
[0018] 2) Dissolve 42g of potassium acetate in 220ml of DMF and distill to remove water and a small amount of low-boiling impurities. After the distilled DMF reaches the constant boiling point, cool the mixture to 100°C and then add the reaction product A42g (0.095 mol) and 200ml DMF solution, reacted at 105°C for 5h. TLC tracking detect...
Embodiment 2
[0022] 1) In a 500ml dry reaction flask, add 50g raw material prednisone (0.14mol), 100ml ethyl acetate, 100ml acetic anhydride, 0.48g p-toluenesulfonic acid, install a reflux condenser, stir and heat up under nitrogen protection And the temperature was controlled at 60°C for 4h. After TLC detection, the reaction was cooled to room temperature, ethyl acetate was evaporated, then washed with 150ml of water, filtered, and the filter cake was dried at low temperature to obtain a crude product, which was separated and purified to obtain a white solid powder A (17a, 21-diacetoxy- 1,4-pregnadiene-3,11,20-trione) 53.8g, yield 87%.
[0023] 2) Add 53.8 intermediate A (0.12mol), 250ml DMF, and 27g potassium acetate into a three-necked flask, stir and heat to 90°C for 7 hours, and TLC traces and detects that the reaction is complete. After cooling down to room temperature, the reaction solution was slowly poured into 300ml of ice water with stirring, and an off-white powder was precipi...
Embodiment 3
[0027] 1) In a 500ml dry reaction flask, add 60g raw material prednisone (0.17mol), 120ml ethyl acetate, 120ml acetic anhydride, 0.48g p-toluenesulfonic acid, install a reflux condenser, stir and heat up under nitrogen protection And the temperature was controlled at 80°C for 2h. After TLC detection, the reaction was cooled to room temperature, ethyl acetate was evaporated, then washed with 150ml of water, filtered, and the filter cake was dried at low temperature to obtain a crude product, which was separated and purified to obtain a white solid powder A (17a, 21-diacetoxy- 1,4-pregnadiene-3,11,20-trione) 67.8g, yield 90.3%.
[0028] 2) Add 67.8g of intermediate A (0.154mol), 300ml of DMF, and 67.8g of sodium acetate into a three-neck flask, stir and heat to 90°C for 7 hours, and TLC traces and detects that the reaction is complete. After cooling down to room temperature, the reaction solution was slowly poured into 300ml of ice water with stirring, and an off-white powder w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com