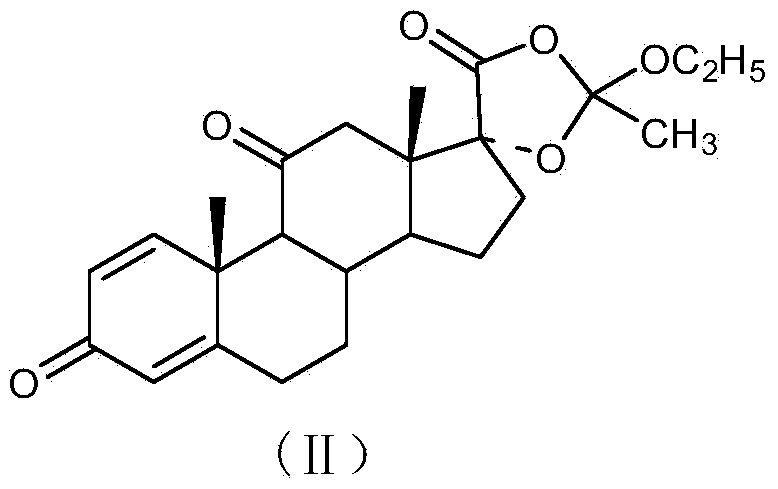
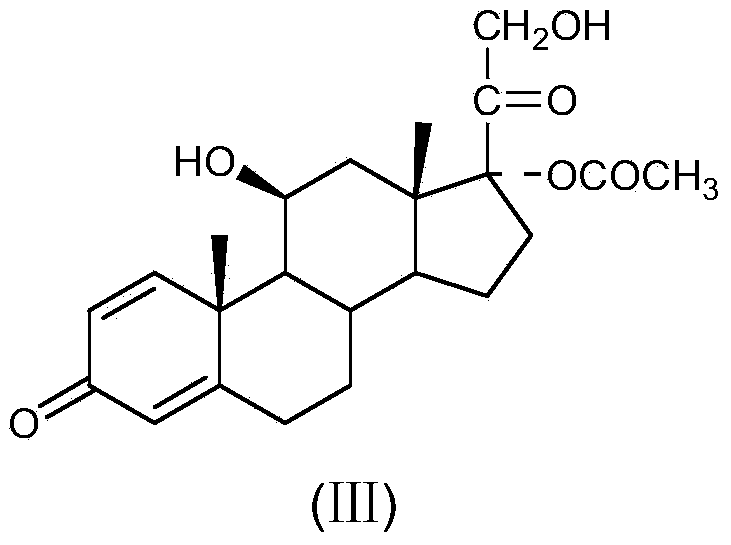
Preparation method of R-budesonide
A single, isomer technology, applied in the field of preparation of adrenal cortex hormone drugs, can solve the problems of unfavorable target products, high production costs, and high toxicity of operators, and achieve good separation effect and high yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
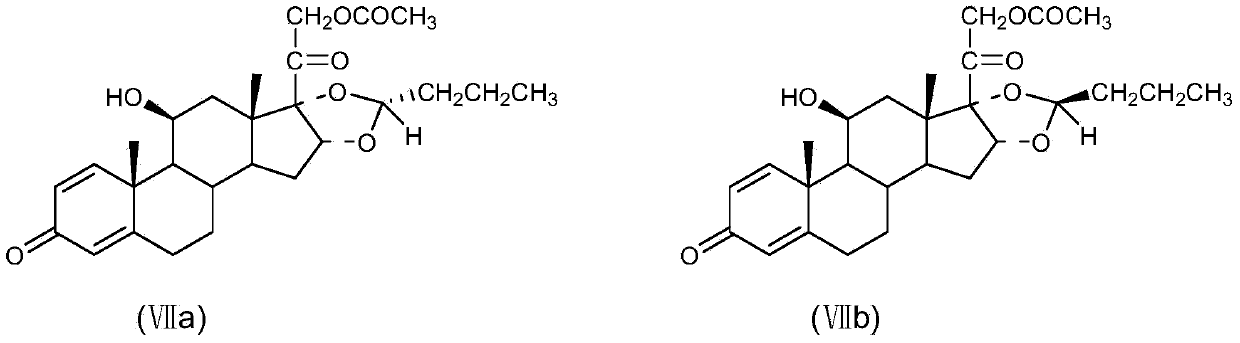
[0037] Put 100 g of the prepared compound of formula VII (R / S=85 / 15) into a reaction flask with mechanical stirring, add 800 ml of methanol, and reflux to dissolve completely. Then it was cooled to room temperature and filtered to obtain 89.0 g of VII.
[0038] R isomer (VIIa): 95.2%; S isomer (VIIb): 4.8%.
Embodiment 2
[0040] Put 100 g of the purified compound of formula VII (R / S=95 / 5) into a reaction flask with mechanical stirring, add 800 ml of methanol, and reflux to dissolve completely. Then it was cooled to room temperature and filtered to obtain 92.0 g of VII.
[0041] R isomer (VIIa): 98.9%; S isomer (VIIb): 1.1%.
Embodiment 3
[0043] Put 100 g of the refined and purified compound of formula VII (R / S=99 / 1) into a reaction flask with mechanical stirring, add 800 ml of methanol, and reflux to dissolve completely. Then it was cooled to room temperature and filtered to obtain 93.6g of VII.
[0044] R isomer (VIIa): 99.6%; S isomer (VIIb): 0.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com