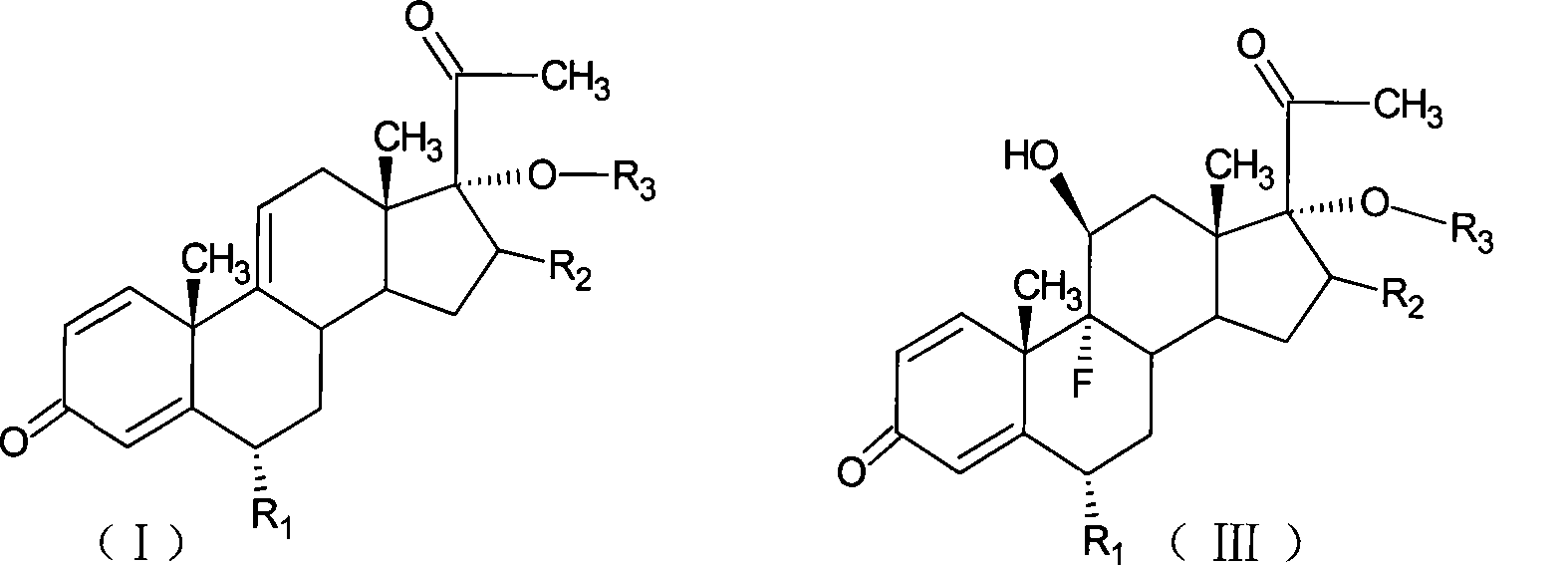
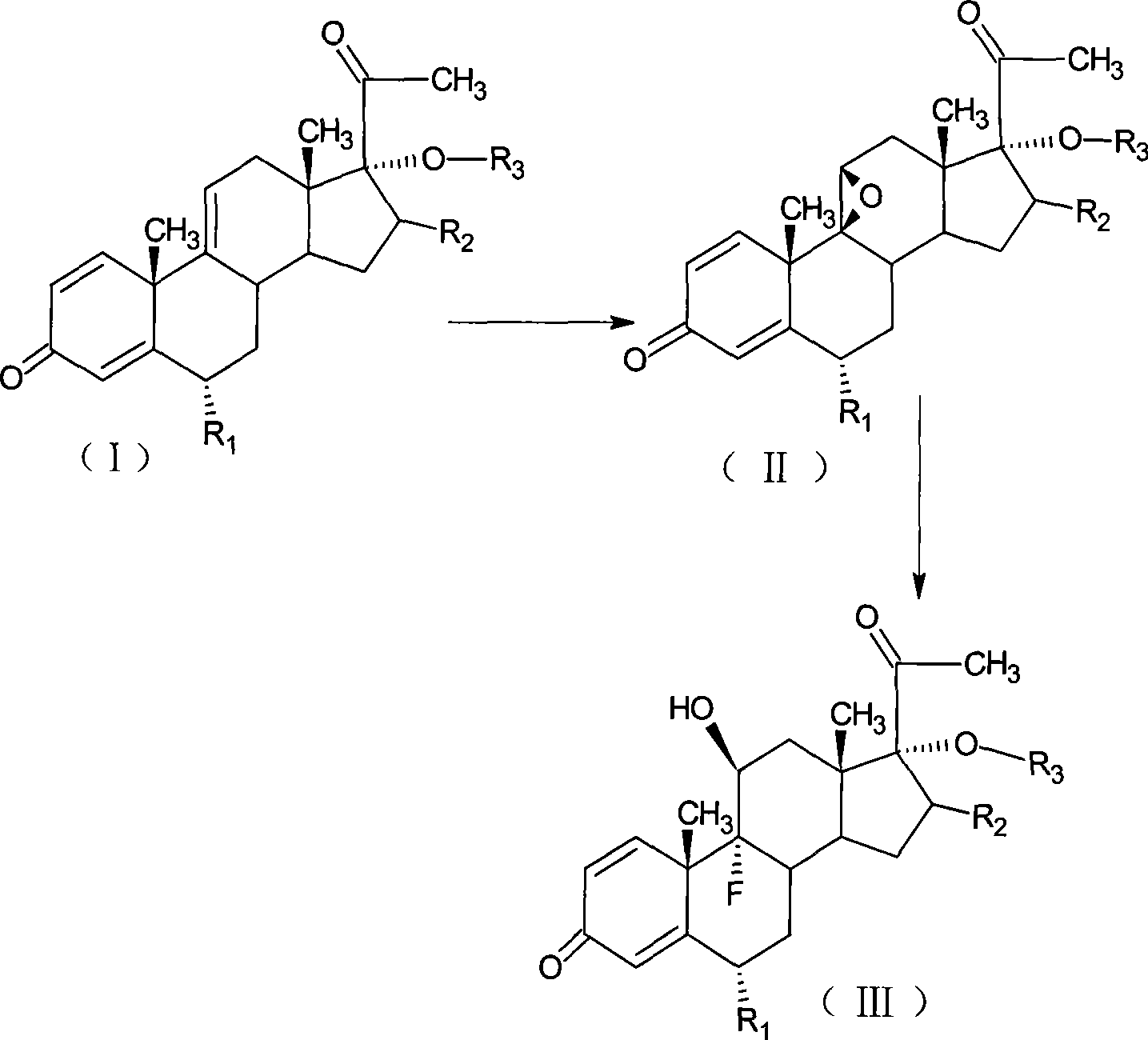
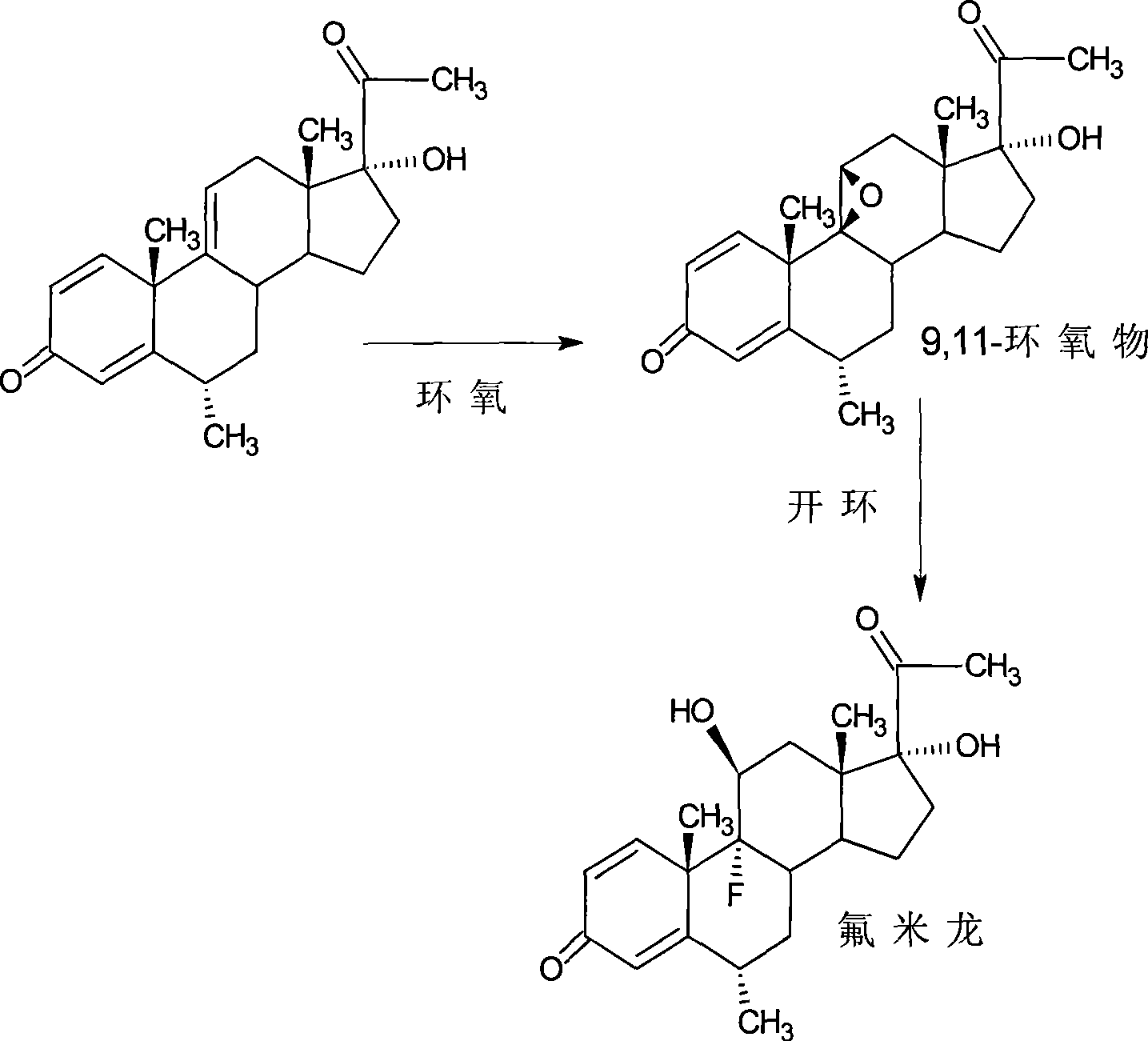
Preparation of fluorometholone and derivatives thereof
A technology of fluorometholone and compounds, which is applied in the field of preparation of steroidal compounds, can solve the problems of increased recrystallization separation intensity, complex fermentation process, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The preparation of embodiment one fluorometholone
[0049] 9,11-epoxy reaction
[0050] Add 10g of 6α-methyl-17α-hydroxyl-1,4,9-triene-pregna-3,20-dione (CN1896090) and 120ml of acetone into the reaction flask, stir, cool to 0°C, and Add 9g of NBS within 30 minutes, keep the reaction at 5-10°C for 2 hours, add 10% sodium carbonate aqueous solution to neutralize to PH=6.5, raise the temperature to 20±2°C, add 10% sodium hydroxide aqueous solution 15ml within 1 hour , controlled temperature at 20-25°C for 2 hours, neutralized with acetic acid to PH = 7, concentrated under reduced pressure until there was no acetone smell, diluted into ice water, filtered, and dried to obtain 11.2 g of 9,11-epoxide.
[0051] 9,11-ring opening reaction
[0052] Add 11.2g of 9,11-epoxy compound obtained by the reaction of 9,11-epoxy, 60ml of DMF into the reaction bottle, stir, cool down to -5°C, pass in hydrogen fluoride gas, and keep it at -5~0°C for 1 hour , diluted in ice water, adjust...
Embodiment 2
[0053] The preparation of embodiment two fluorometholone
[0054] 9,11-epoxy reaction
[0055] Add 10g of 6α-methyl-17α-hydroxyl-1,4,9-triene-pregna-3,20-dione (CN1896090) and 50ml of tetrahydrofuran into the reaction flask, stir, cool to 0°C, and Add 9g of NCS within 30 minutes, keep the reaction at 5-10°C for 2 hours, dilute in water, filter to obtain the halide wet product, and directly dissolve the solid in 100ml of acetone, raise the temperature to 20±2°C, and add 10% within 1 hour Potassium hydroxide aqueous solution 15ml, temperature control 20-25°C, react for 2 hours, neutralize with acetic acid to PH=7, concentrate under reduced pressure until there is no acetone smell, dilute into ice water, filter and dry to obtain 9,11-epoxy compound 10.9 g.
[0056] 9,11-ring opening reaction
[0057]Add 10.9g of 9,11-epoxy compound obtained by the reaction of 9,11-epoxy, 50ml of tetrahydrofuran into the reaction bottle, stir, cool down to -5°C, add 50ml of 47% hydrogen fluorid...
Embodiment 3
[0058] The preparation of embodiment three fluorometholone-17-acetates
[0059] 17-position esterification reaction
[0060] Add 10g of 6α-methyl-17α-hydroxyl-1,4,9-triene-pregna-3,20-dione (CN1896090) into the reaction flask, add 25ml of anhydrous acetic anhydride and 25ml of acetic acid, add 2.5 g of p-toluenesulfonic acid, kept at 75±2°C for 1 hour, diluted in ice water, filtered, and dried to obtain 11.1 g of 6α-methyl-17α-hydroxyl-1,4,9-triene- Pregna-3,20-dione-17-acetate.
[0061] 9,11-epoxy reaction
[0062] Add 11.1g of 6α-methyl-17α-hydroxyl-1,4,9-triene-pregna-3,20-dione-17-acetate, 50ml of tetrahydrofuran into the reaction flask, stir, and cool to 0 ℃, add 6.5 g of dibromocyanoacetamide within 30 minutes, keep the reaction at 5-10 ℃ for 2 hours, dilute in water, filter to obtain the halide wet product, and directly dissolve the solid in 40ml of methanol and 30ml of dichloromethane, Raise the temperature to 20±2°C, add 15ml of 10% potassium hydroxide aqueous sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com