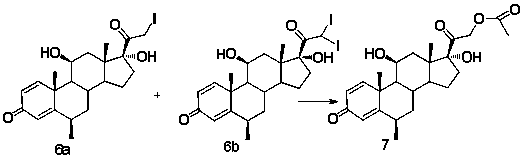
Method for preparing 6 beta-methylprednisolone
A technology of methylprednisolone and methyl, which is applied in the field of chemical preparation, can solve the problem of the difficulty in ensuring the purity and pharmacological activity of methylprednisolone, and the inability to prepare 6β-methylprednisolone reference substances, etc. problem, to achieve the effect of improving purity and pharmacological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The first step, Grignard, hydrolysis, and elimination: In a 250mL dry reaction bottle, add tetrahydrofuran (53mL, 5.3V), then add magnesium flakes (6.7g, 0.67W), pass through monochloromethane until the temperature rises rapidly to 55-61 °C, lower the temperature until the reaction is stable. Add tetrahydrofuran (80mL, 8V) into the reaction flask, keep warm at 30-50°C and ventilate until the magnesium dissolves, then ventilate for 6-8 hours, then inject about 20g of monochloromethane (20g, 2W), and store in airtight. Then concentrate under normal pressure and distill off tetrahydrofuran until the temperature rises to 71-74°C. After cooling down to room temperature, it was protected with nitrogen gas and sealed for use. Add tetrahydrofuran (20mL, 2V) into a 250mL reaction flask, add 6-methylepoxide 1 (10g, 1W), stir for 20-30 minutes, then slowly add Grignard reagent (60mL, 6V), heat and reflux for 4 ~5 hours, cool to below 20°C; add glacial acetic acid (20mL, 2V), ice...
Embodiment 2
[0050] The first step, Grignard, hydrolysis, and elimination: In a 250mL dry reaction bottle, add tetrahydrofuran (53mL, 5.3V), then add magnesium flakes (6.7g, 0.67W), pass through monochloromethane until the temperature rises rapidly to 55-61 °C, lower the temperature until the reaction is stable. Add tetrahydrofuran (80mL, 8V) into the reaction flask, keep warm at 30-50°C and ventilate until the magnesium dissolves, then ventilate for 6-8 hours, then inject about 20g of monochloromethane (20g, 2W), and store in airtight. Then concentrate under normal pressure and distill off tetrahydrofuran until the temperature rises to 71-74°C. After cooling down to room temperature, it was protected with nitrogen gas and sealed for use. Add tetrahydrofuran (20mL, 2V) into a 250mL reaction flask, add 6-methylepoxide 1 (10g, 1W), stir for 20-30 minutes, then slowly add Grignard reagent (60mL, 6V), heat and reflux for 4 ~5 hours, cool to below 20°C; add glacial acetic acid (20mL, 2V), ice...
Embodiment 3
[0053] The first step, Grignard, hydrolysis, and elimination: In a 250mL dry reaction bottle, add tetrahydrofuran (53mL, 5.3V), then add magnesium flakes (6.7g, 0.67W), pass through monochloromethane until the temperature rises rapidly to 55-61 °C, lower the temperature until the reaction is stable. Add tetrahydrofuran (80mL, 8V) into the reaction flask, keep warm at 30-50°C and ventilate until the magnesium dissolves, then ventilate for 6-8 hours, then inject about 20g of monochloromethane (20g, 2W), and store in airtight. Then concentrate under normal pressure and distill off tetrahydrofuran until the temperature rises to 71-74°C. After cooling down to room temperature, it was protected with nitrogen gas and sealed for use. Add tetrahydrofuran (20mL, 2V) into a 250mL reaction flask, add 6-methylepoxide 1 (10g, 1W), stir for 20-30 minutes, then slowly add Grignard reagent (60mL, 6V), heat and reflux for 4 ~5 hours, cool to below 20°C; add glacial acetic acid (20mL, 2V), ice...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com