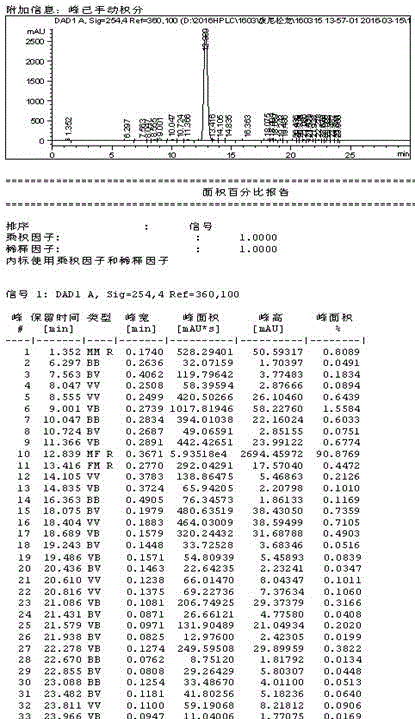
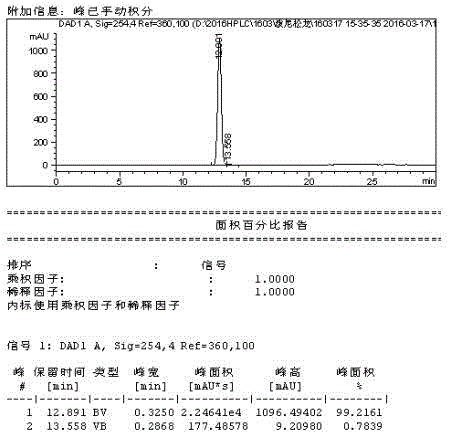
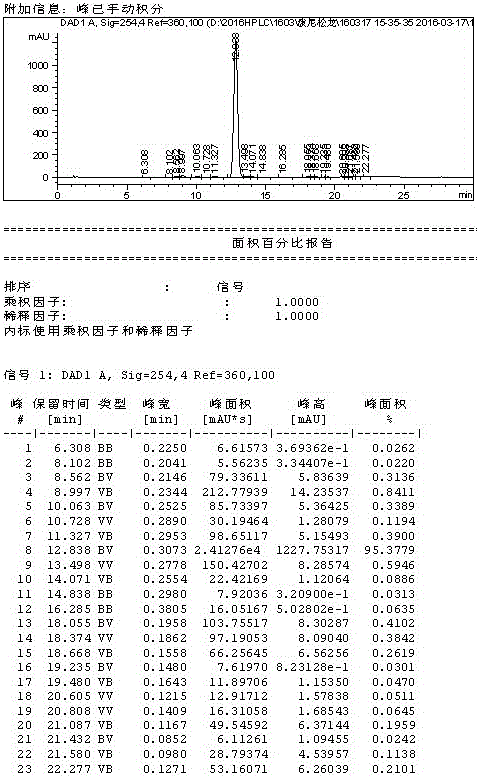
Method for preparing prednisolone
A technology for prednisolone and products, which is applied in the field of chemical preparation, can solve the problems of long process route, unfriendly environment, and many by-products of prednisolone, and achieves low cost, short process route, and improved selectivity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] The first step, ethylene glycol protection reaction: add ethylene glycol (150mL, 2.70mol) and triethyl orthoformate (300mL, 1.80mol), cyclohexane 600mL and p-toluenesulfonic acid (1.5g , 9.48mmol), heated to reflux, reacted for 2 hours, distilled ethanol-cyclohexane azeotrope at normal pressure, added 300mL cyclohexane when the solvent was evaporated, refluxed for 1 hour, and distilled ethanol-cyclohexane azeotrope at normal pressure Ethane azeotrope, when no solvent is evaporated, heated to 100°C, distilled under reduced pressure for 2 hours, cooled to room temperature to obtain active ester, protected by nitrogen for use. Under the protection of nitrogen, add dihydroxyprogesterone dehydrogenate 1 (10 g, 29.1 mmol) into a 500 mL reaction flask, add 100 mL of tetrahydrofuran and 10 mL of concentrated hydrochloric acid, add 10 mL of active ester at room temperature, and react at 25 °C for 3 hours. Stop the reaction when there is no raw material point detected by TLC (thi...
Embodiment 2
[0067] The first step, propylene glycol protection reaction: (1) Add 1,3-propanediol (150mL, 2.08mol), triethyl orthoformate (150mL, 0.90mol), 400mL cyclohexane and p-toluenesulfonic acid in a 2000mL reaction flask (1.5g, 8.71mmol), heated to reflux, reacted for 2 hours, fractionated ethanol-cyclohexane azeotrope at normal pressure, added 200mL of cyclohexane when no solvent was evaporated, refluxed for 1 hour, fractionated at normal pressure Ethanol-cyclohexane azeotrope, when no solvent is evaporated, heated to 100°C, distilled under reduced pressure for 2 hours, cooled to room temperature to obtain active ester, protected by nitrogen for use. (2) Under nitrogen protection, add dihydroxyprogesterone dehydrogenate 1 (10g, 29.1mmol), 291mL tetrahydrofuran and 10mL phosphoric acid in a 500mL reaction flask, add 12mL active ester at room temperature, and react at 30°C for 6 hours. Stop the reaction when there is no raw material point detected by TLC, add saturated aqueous sodium...
Embodiment 3
[0070] The first step, neopentyl glycol protection reaction: (1) Add neopentyl glycol (150mL, 1.53mol), triethyl orthoformate (450mL, 2.70mol), cyclohexane 800mL and p-toluene into a 2000mL reaction flask Sulfonic acid (4.5g, 26.13mmol), heated to reflux, reacted for 2 hours, distilled ethanol-cyclohexane azeotrope at normal pressure, added 400mL cyclohexane when no solvent was evaporated, refluxed for 1 hour, normal pressure Fractionally distill the ethanol-cyclohexane azeotrope, and when no solvent is evaporated, heat to 100°C, distill under reduced pressure for 2 hours, and cool to room temperature to obtain the active ester, which is protected under nitrogen for use. (2) Under nitrogen protection, add dihydroxyprogesterone dehydrogenate 1 (10g, 29.1mmol), 60mL tetrahydropyran and 10mL perchloric acid in a 250mL reaction bottle, add 8mL active ester at room temperature, and react at 20°C for 4 Hour. Stop the reaction when there is no raw material point detected by TLC, add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com