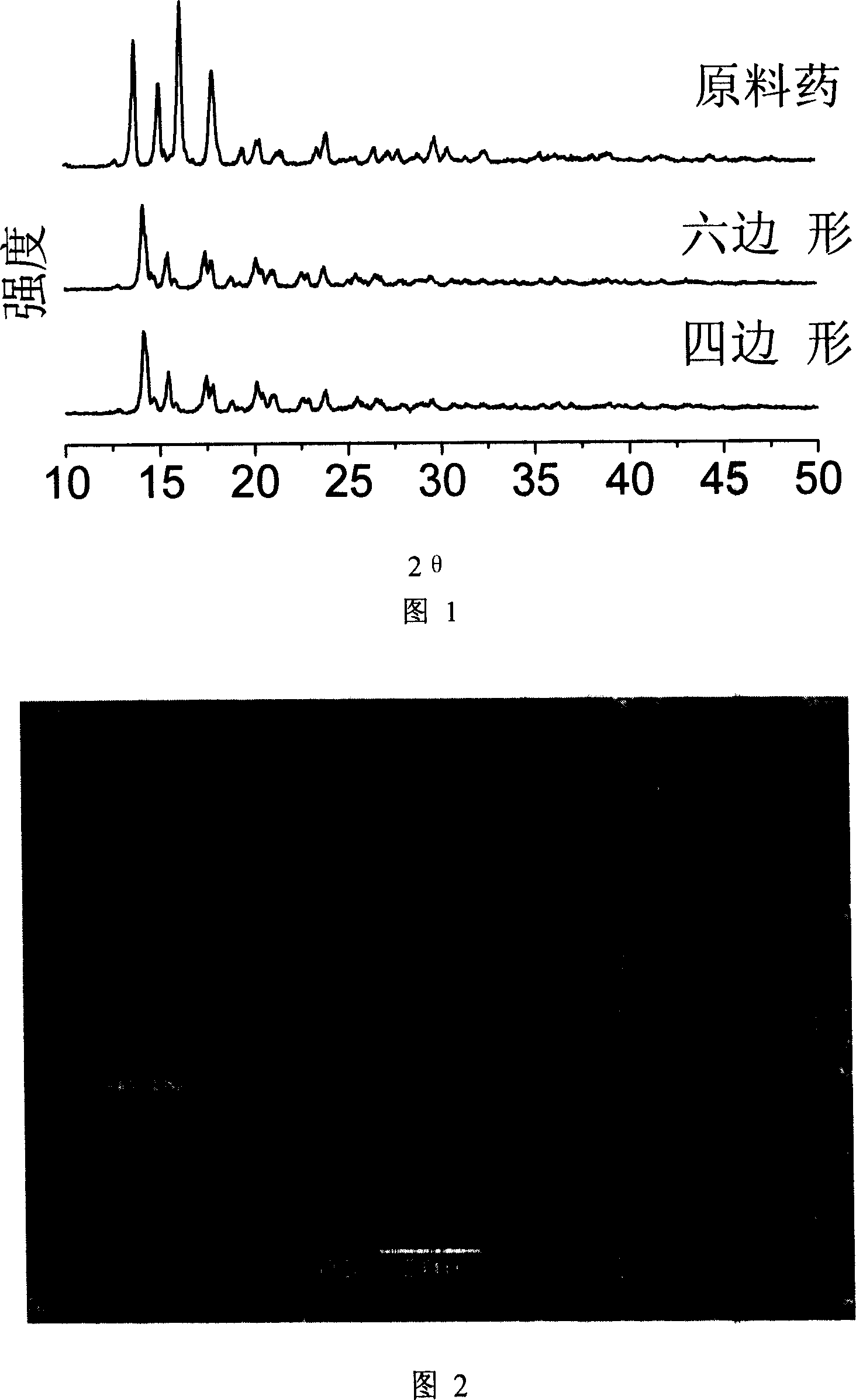
Preparation of superfine prednisolone powder
A technology of prednisolone and powder, which is applied in the field of drug micronization, can solve the problems of complex and unstable operating equipment, and achieve the effects of reducing settling time, increasing driving force and reducing requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Take prednisolone crude drug 5.2g, dissolve and prepare clear prednisolone solution with 5ml N-methylpyrrolidone. Prepare 500ml of deionized water in a 1000ml beaker. Place the beaker containing the 500ml anti-solvent solution under the emulsifier at a speed of 10000rpm, then inject the above-mentioned solution containing 5.2g of prednisolone into the stirring anti-solvent, and the two solutions are mixed very quickly and efficiently to produce Precipitation and crystallization generate white prednisolone precipitates, and the obtained slurry is filtered, washed, and dried to obtain prednisolone granules. In operation, the volume ratio of prednisolone drug solution to aqueous solution is about 1:100, and when the precipitation temperature is 2 or 30°C, the morphology and particle size obtained by the two temperatures are basically the same, that is, the length is 5-20 μm, and the width is 2- 3μm rod-shaped particles, as shown in Figure 3.
Embodiment 2
[0035] Take prednisolone crude drug 5.2g, dissolve and prepare clear prednisolone solution with 5ml N-methylpyrrolidone. Prepare 500ml of 0.2wt% hydroxypropyl methylcellulose aqueous solution in a 1000ml beaker. Place the beaker containing the 500ml anti-solvent solution under the emulsifier at a speed of 10000rpm, then inject the above-mentioned solution containing 5.2g of prednisolone into the stirring anti-solvent, and the two solutions are mixed very quickly and efficiently to produce Precipitation and crystallization generate white prednisolone precipitates, and the obtained slurry is filtered, washed, and dried to obtain prednisolone granules. In operation, the volume ratio of prednisolone drug solution and 0.2wt% hydroxypropyl methylcellulose aqueous solution is about 1: 100, and when precipitation temperature is 14 ℃, what obtain is approximate hexagonal flake particle; At 4°C, approximately quadrilateral particles are obtained. Its average particle size is about 2 μ...
Embodiment 3
[0037] Take prednisolone crude drug 2g, dissolve and prepare clear prednisolone solution with 5ml dimethyl sulfoxide. Prepare 200ml of 0.25wt% hydroxypropylcellulose aqueous solution in a 500ml beaker. Place the beaker containing the 500ml anti-solvent solution under the emulsifier at a speed of 10000rpm, then inject the above solution containing 2g of prednisolone into the stirring anti-solvent, the two solutions are mixed very quickly and efficiently and precipitate Crystallization produces white prednisolone precipitates, and the resulting slurry is filtered, washed, and dried to obtain prednisolone granules. In operation, the volume ratio of prednisolone drug solution and 0.25wt% hydroxypropyl cellulose aqueous solution is about 1: 40, and when precipitation temperature is 14 ℃, what obtain is hexagonal sheet-shaped particle; Precipitation temperature is 4 ℃ When , quadrilateral particles are obtained. Its average particle size is about 3 μm, and at least 90% of the part...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com