Instant gelling agent for treating allergic rhinitis
An anti-allergic, gel technology, applied in allergic diseases, medical preparations containing active ingredients, organic active ingredients, etc., can solve the problem of high viscosity, affecting the accuracy of dosage, and clinical efficacy question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1. Prescription screening of ion-sensitive instant gel for nasal use
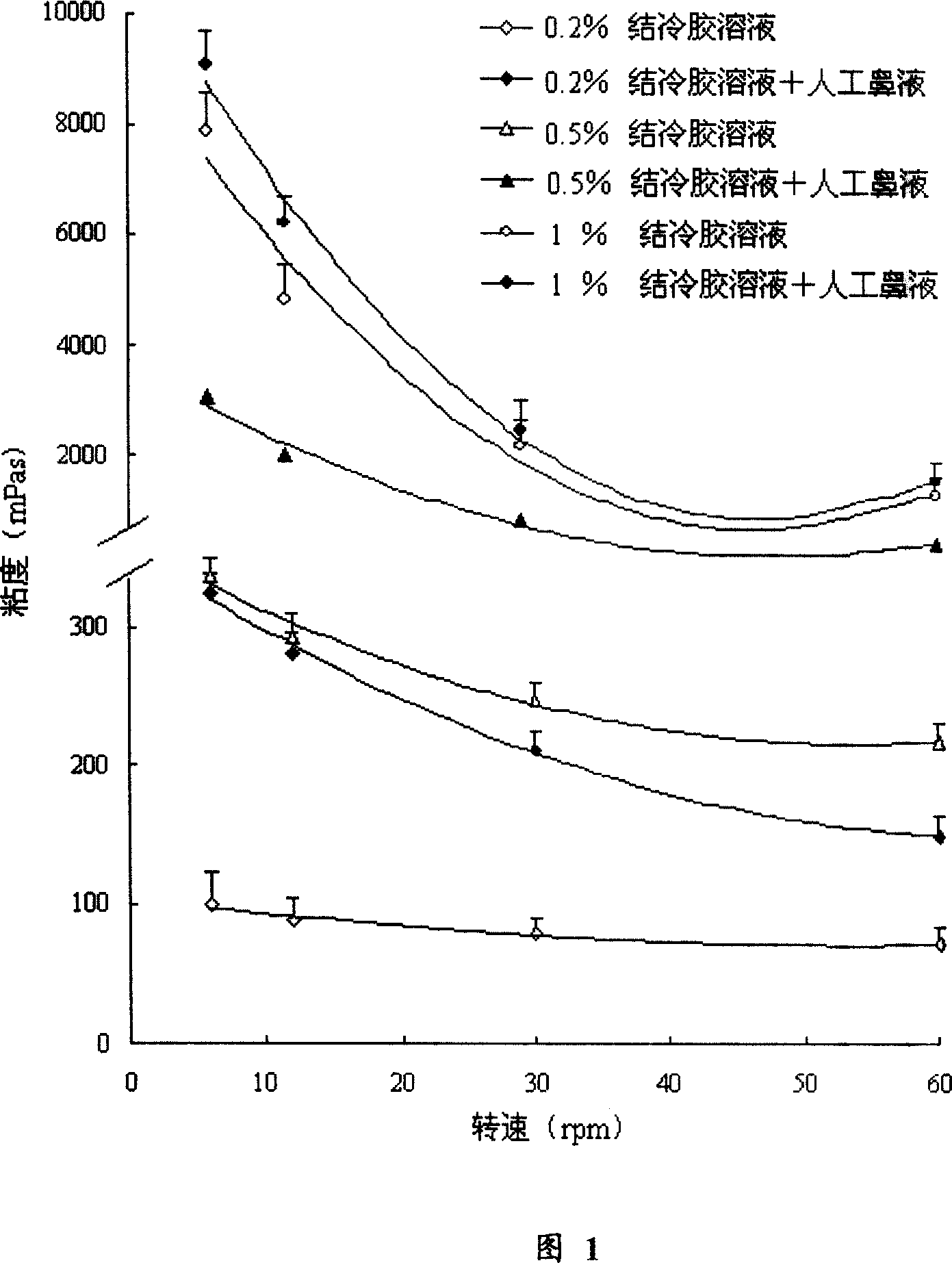
[0032] Taking the viscosity and suspension-sedimentation ratio of the formulation as indicators, and using the method of single-factor investigation, the formulations of ion-sensitive instant gel for nasal use were screened and determined, as shown in Table 1.
[0033] Table 1 The prescription (w / w%) of ion-sensitive nasal instant gel
[0034] Prescription 1
Prescription 2
Prescription 3
Prescription 4
Prescription 5
Prescription 6
Monohydrate
0.5%
0.05%
1.0%
0.01%
0.2%
0.5%
deacetylated gellan gum
0.5%
0.4%
1%
0.2%
sodium alginate
1%
6%
suspending agent
0.15%
Methylcellulose
3%
5%
HPMC
0.1%
HPMC
0.45%
Methylcellulose
2.5%
...
Embodiment 2
[0054] 1. Prescription screening of temperature-sensitive nasal ready-to-use gel
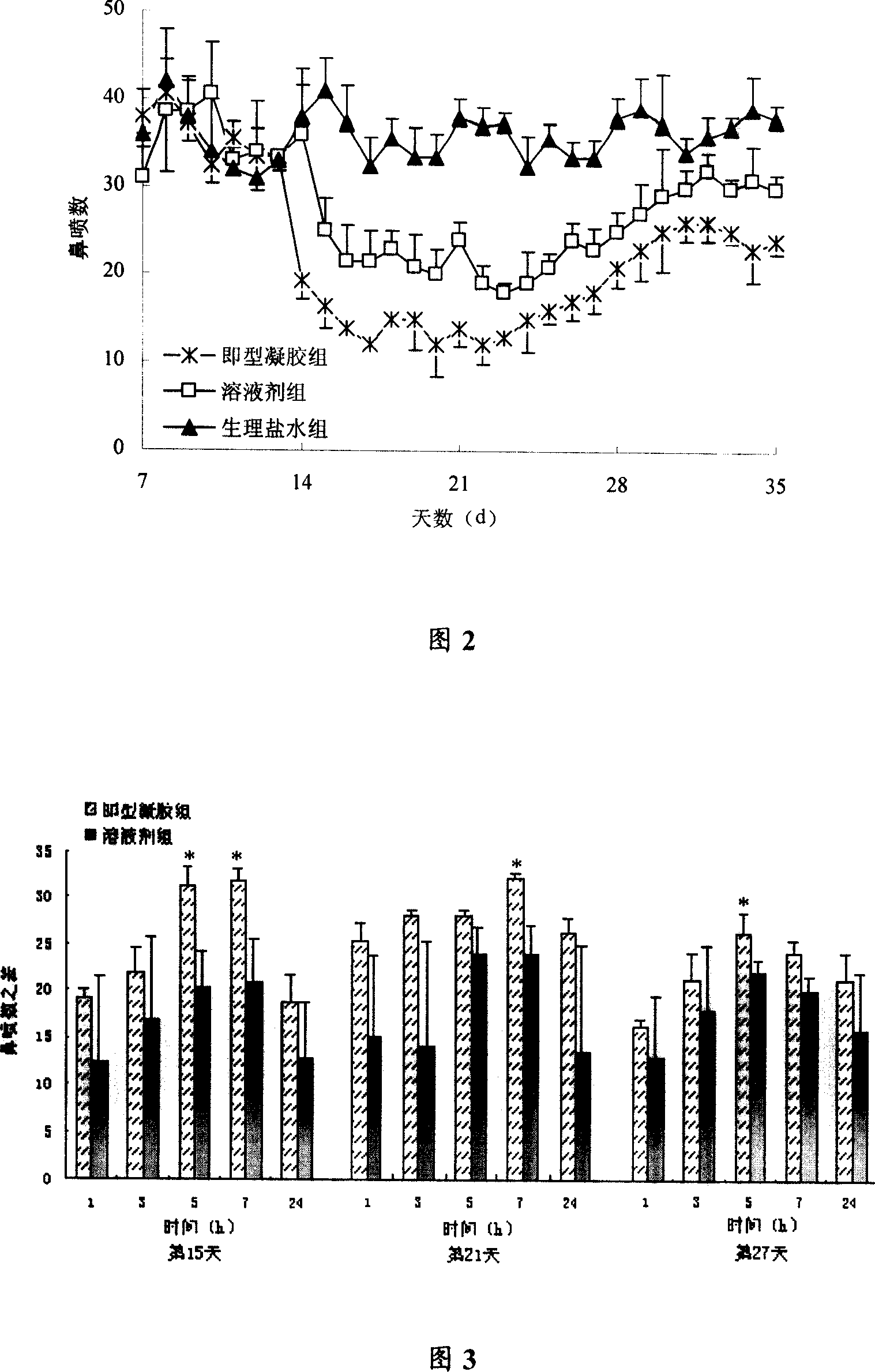
[0055] Taking the viscosity of the preparation and the suspension-sedimentation ratio as indicators, the single-factor investigation method was used to screen and determine the prescription of the temperature-sensitive nasal instant gel as shown in Table 2.
[0056] Table 2 Prescription (w / w%) of temperature-sensitive nasal instant gel
[0057] Prescription 14
Prescription 15
Prescription 16
Prescription 17
Monohydrate
0.1%
1.0%
0.15%
0.5%
Pluronic F127
20%
20%
10%
Pluronic F68
10%
15%
30%
suspending agent
HPMC0.5%
Methylcellulose 3%
Xanthan Gum 0.5%
Western tragacanth 2%
Glycerin 15%
Propylene Glycol 10%
Tween-65 0.2%
Tween-85 0.4%
preservatives
Chlorhexidine acetate 0.0...
Embodiment 3
[0074] 1. Prescription screening of pH-sensitive instant gel for nasal use
[0075] Taking the viscosity of the preparation and the suspension-sedimentation ratio as indicators, the single-factor investigation method was used to screen and determine the prescription of the pH-sensitive instant gel for nasal use as shown in Table 4.
[0076] Table 4 pH-sensitive nasal instant gel preparation prescription (w / w%)
[0077] Prescription 24
Prescription 25
Prescription 26
Prescription 27
Prescription 28
Prescription 29
mometasone furoate
Monohydrate
0.02%
0.55%
1.0%
0.5%
0.1%
0.1%
CAP
40%
10%
AEA
9%
1%
Carbopol 940P
0.65%
0.59%
suspending agent
HPMC
HPMC
CMC-Na
CMC-Na
HPMC
HPMC
[0078] 0.5%
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| phase transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com