Improvements Relating To Inhalable Particles
a technology of inhalable particles and powder formulations, which is applied in the direction of powder delivery, medical preparations, pharmaceutical active ingredients, etc., can solve the problems of inability to direct prepare the particle sizes required for unhelpful crystalline habits, and inability to achieve the effect of effective drug deposition in the central and deep lung compartments, easy inhalation, and reduced dosag
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples 1 to 33
Tolterodine L-Tartrate / Examples C1 to C5
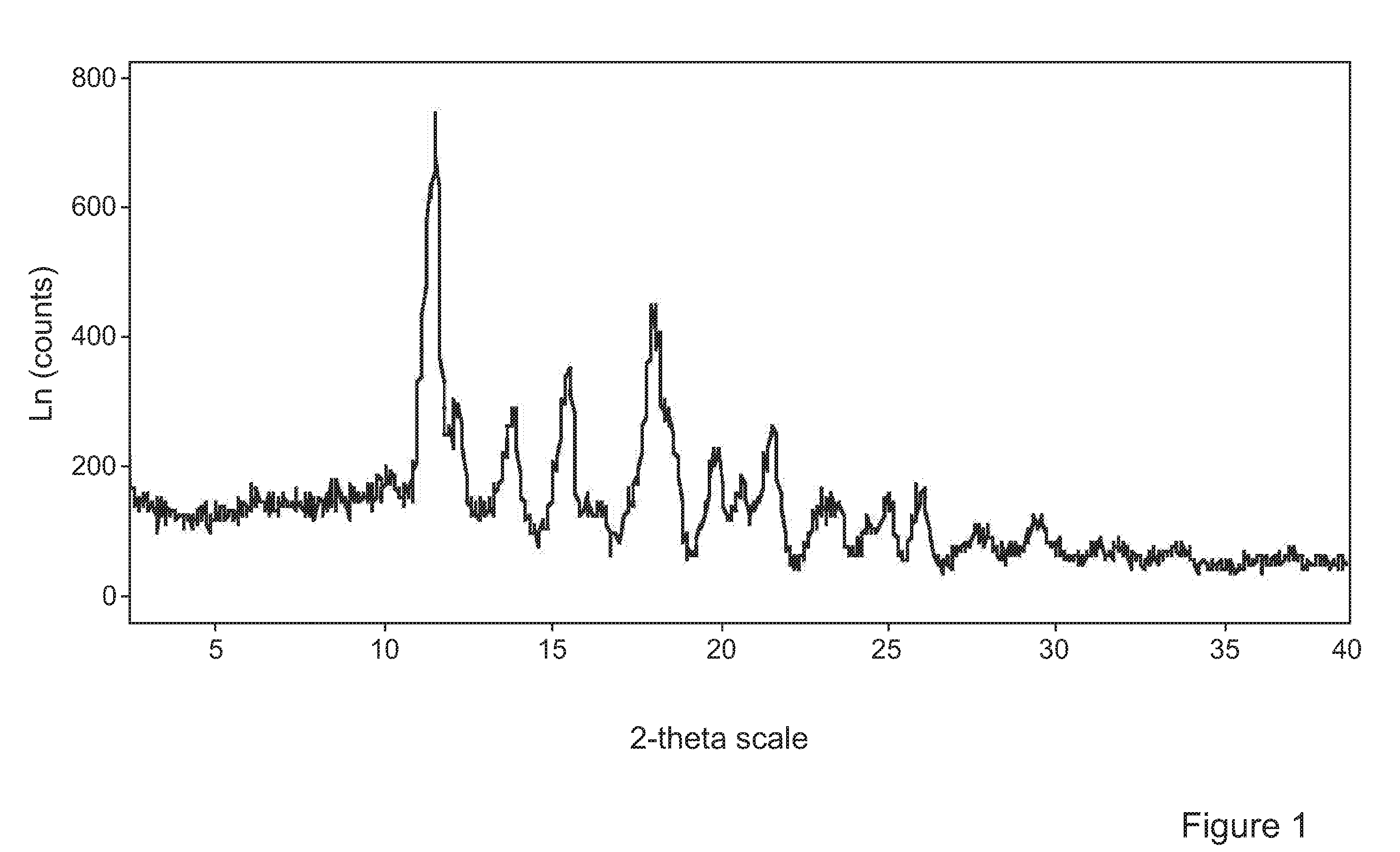
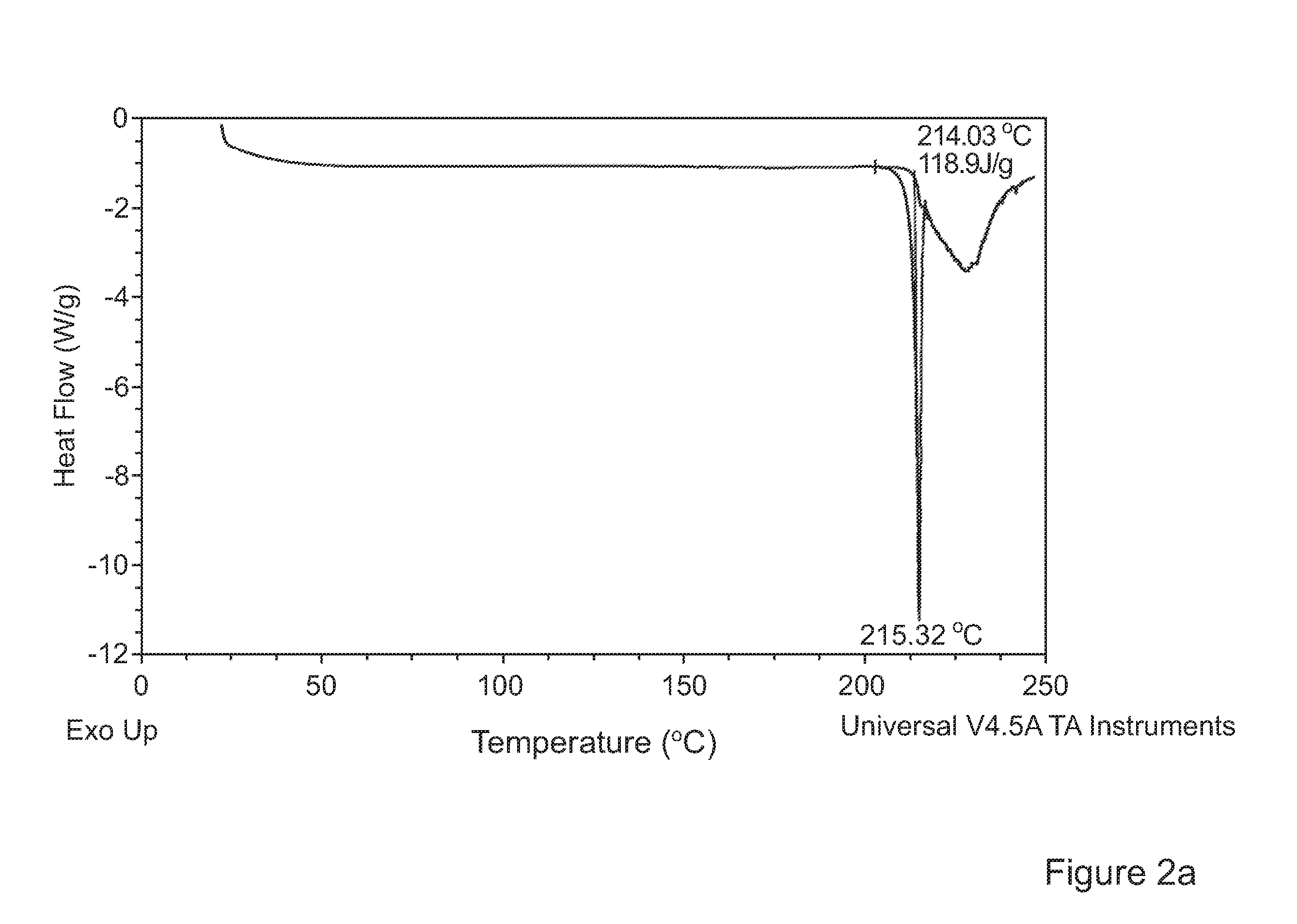
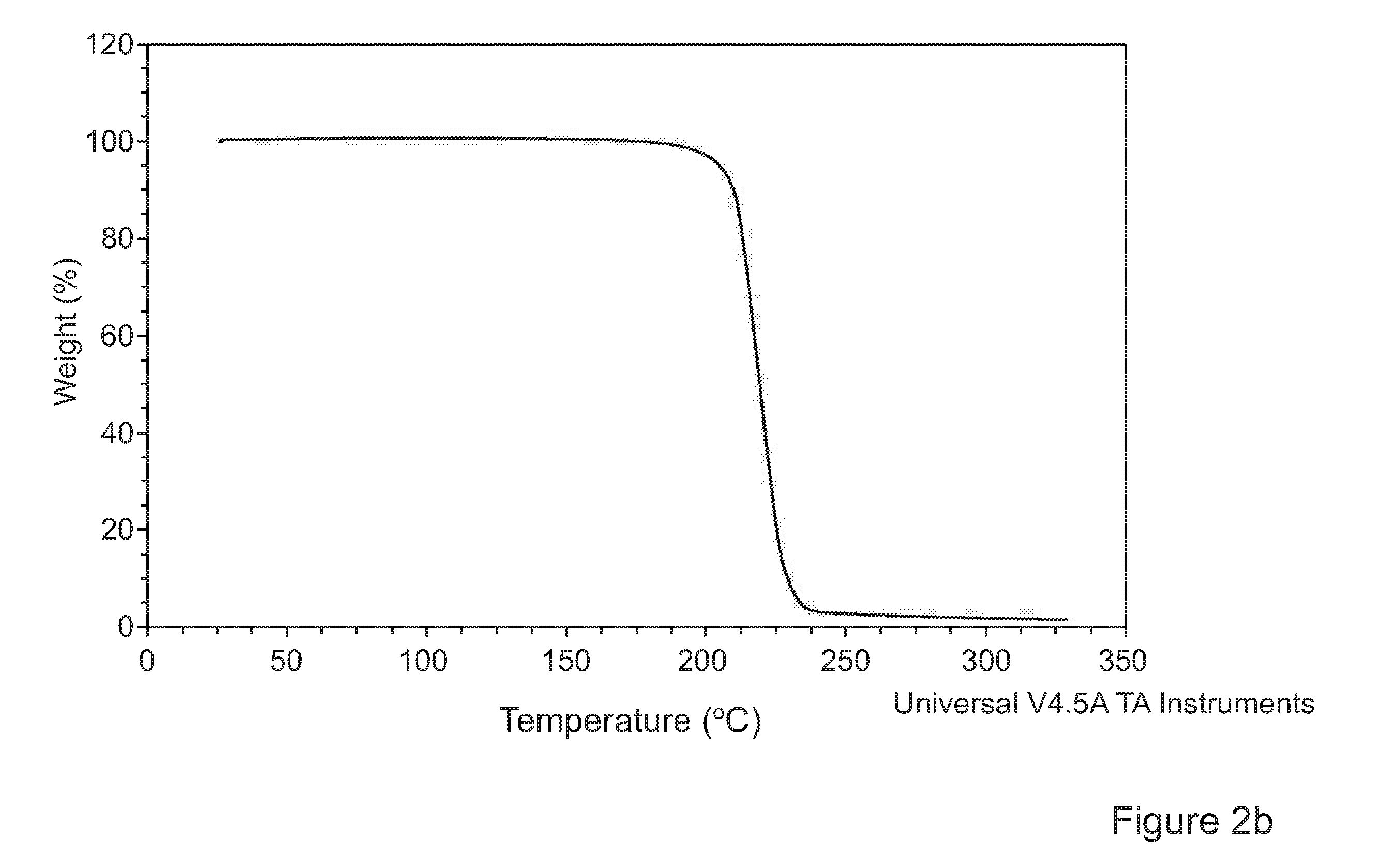
[0096]Experiments were conducted with the object of forming advantageously inhalable or insufflable particles of Tolterodine L-tartrate using supercritical anti-solvent (SAS) precipitation.
[0097]Tolterodine L-tartrate forms acicular (needle-like) crystals. Acicular particles grow extremely fast in one dimension. This presents particular challenges in the context of precipitation of inhalable or insufflable particles. Specific particle formation conditions for providing readily inhalable or insufflable particles, in particular particles with D50 and D90 percentiles between 1 to 4 μm and 2 to 10 μm and volume mean diameter<7 μm, more preferably <5 μm respectively were investigated.
[0098]The method used to generate particles of inhalable tolterodine L-tartrate was a SAS (Supercritical Anti-Solvent) process
[0099]For each example, tolterodine L-tartrate was dissolved in methanol.
[0100]A stream of the solution of tolterodine L-tartrate was contacte...
example 34
Hydroxymethyl Tolterodine
[0137]A further experiment was conducted to prepare inhalable particles of hydroxymethyl tolterodine.
[0138]Hydroxymethyl tolterodine was precipitated, in the general manner of Examples 1 to 33, from acetonitrile solution (50.0 mg / ml) using a carbon dioxide temperature of 40° C., a carbon dioxide pressure of 85 bar, a carbon dioxide density of 0.353, a hydroxymethyl tolterodine solution flow of 0.32 g / min and carbon dioxide flow of 36 g / min. The product was a fine, free flowing powder that had D50 and D90 percentiles of 1.2 μm and 3.1 μm and volume mean diameter 1.5 μm respectively. The sample was amorphous by PXRD.
example 35
Batch Consistency
[0139]The results of reproducibility of seven batches of tolterodine L-tartrate produced using the same conditions as in Example 1 are shown in FIG. 6 in the form of a particle size distribution curve. Particle size analysis of the product was carried out using a Sympatec Helos Particle Size Analyser using an aerosolisation pressure of 4 bar.
[0140]Particles possessed a median diameter of D50=1.4 μm with a percentiles diameter of D90=3.3 μm and a volume mean diameter (VMD)=1.7 μm. These data demonstrate the excellent reproducibility under the conditions of Example 1 process and confirm the suitability of the particle size distribution for respiratory drug delivery applications.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Particle size distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com